Efficacy for savolitinib in patients with MET exon 14 alterations is in line with that for other MET tyrosine kinase inhibitors, but safety profile is different
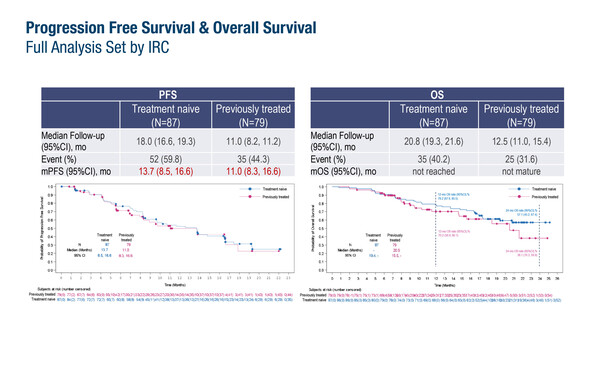
In a confirmatory Chinese phase IIIb trial presented at the European Lung Cancer Congress 2024 (Prague, Czech Republic, 20–23 March), the selective MET tyrosine kinase inhibitor (TKI), savolitinib, was associated with a median progression-free survival (PFS) of 13.7 months (95% confidence interval [CI] 8.5–16.6) in 87 treatment-naïve patients with advanced non-small cell lung cancer (NSCLC) harbouring a MET exon 14 mutation (Abstract 1MO). The objective response (ORR) and disease control rates (DCR) were 62.1% and 92.0%, respectively. Among 79 previously treated patients, the median PFS was 11.0 months (95% CI 8.3–16.6), with ORR and DCR of 39.2% and 92.4%, respectively. Median overall survival (OS) was not reached (treatment-naïve population) or not mature (previously treated population).
Altogether, 60.2% of all patients had grade ≥3 treatment-related treatment-emergent adverse events (TRAEs), mainly abnormal hepatic function (16.9%), increased alanine aminotransferase (14.5%) and increased aspartate aminotransferase (12.0%). In total, TRAEs led to dose reductions in 65.7% of patients and led to discontinuation in 8.4%. In both the treatment-naïve and previously treated populations, patients were generally elderly (median age of around 70 years) with adenocarcinoma (80.5% and 78.5%, respectively) or pulmonary sarcomatoid carcinoma (8.0% and 5.1%, respectively) and 11.5% and 26.6%, respectively, had brain involvement.
“In treatment-naïve populations, the efficacy results from this trial are in line with those demonstrated with other MET TKIs currently available, namely tepotinib (N Engl J Med. 2020;383:931–943) and capmatinib (N Engl J Med. 2020;383:944–957), and others,” says Dr Jordi Remon from Gustave Roussy, Villejuif, France. “It is important to note that the trial included 5–8% of patients with sarcomatoid histology NSCLC, which is a poor prognostic indicator and generally associated with a higher incidence of this MET alteration, around 22% (Front Oncol. 2022:12:1017026) compared with the 3% expected in NSCLC adenocarcinoma,” he comments. “The safety profile of savolitinib is slightly different from other MET TKIs as abnormal hepatic function was the most common toxicity compared with peripheral oedema with other MET TKIs. Dose reductions occurred in two-thirds of patients, which might suggest that initial dosing should be optimised.” Regarding brain involvement, Remon comments, “It would be useful to know if this agent showed activity against the brain metastases reported, because effective intracranial penetration is important when up to one-quarter of patients treated can be expected to have such lesions.” According to Remon, this trial and others have strongly shown that MET TKIs have higher activity when used first line. “Together with the knowledge that eligible patients are often elderly and less able to tolerate standard chemoimmunotherapy, this questions the decision by some regulatory authorities to restrict the use of tepotinib and capmatinib to the subsequent-line setting,” he notes.
MET amplification is an important mediator of acquired resistance in patients with EGFR-mutated NSCLC treated with an EGFR TKI, as well as in other targetable oncogene drivers. In another Chinese study presented in Prague, fluorescence in situ hybridisation (FISH)-detected MET amplification in 43 of 116 (37.1%) patients who progressed on EGFR TKIs, with focal amplification detected in 20.7% and polysomy in 16.4% (Abstract 21P). Using FISH as the reference, detection of focal MET amplification by next-generation sequencing (NGS) showed respective sensitivity, specificity and agreement rates of 66.7%, 98.6% and 90.7% in tissue, and 29.2%, 94.5% and 78.4% in plasma.
Remon comments: “This study shows that true MET amplification was evident in around one-fifth of patients developing TKI resistance although the investigators do not provide a definition for amplification and patient numbers were limited. We are increasingly seeing this mechanism of resistance and the study suggests that NGS could be an alternative strategy to FISH, more so in tissue than in liquid biopsy.” Looking ahead, he notes that savolitinib is being compared with platinum-based doublet chemotherapy in the phase III SAFFRON trial in patients with MET-overexpressed and/or amplified NSCLC who have progressed on first- or second-line osimertinib, with primary results expected in 2025 (NCT05261399).
Abstracts discussed:
Lu S, et al. A phase 3b study of savolitinib in patients with locally advanced or metastatic NSCLC harboring MET exon 14 mutation. European Lung Cancer Congress 2024, Abstract 1MO
Mini Oral Session 1, 20.03.2024, h. 16:30 – 17:35, South Hall 2
Zheng Q. NGS and FISH for MET amplification detection in post EGFR-TKI resistant non-small cell lung cancer (NSCLC) patients: a prospective, multi-center study in China. European Lung Cancer Congress 2024, Abstract 21P
Poster Display Session, 22.03.2024, h. 12:00 – 12:45, Congress Hall Foyer