Although the phase III investigational study with IO102-IO103 plus pembrolizumab is negative, it offers precious insights to advance research in the field
The combination of IO102-IO103 plus pembrolizumab did not lead to significantly improved progression-free survival (PFS) in a phase III trial with 407 patients with unresectable melanoma, according to results presented at the ESMO Congress 2025 (Berlin, 17–21 October) (LBA53). IO102-IO103 is an off-the shelf vaccine targeting PD-L1 and indoleamine 2,3-dioxygenase (IDO) through activation of T cells against both tumour cells and immunosuppressive immune cells expressing PD-L1 and IDO.
In the phase III IOB-013/KN-D18 trial, a numerical increase in median progression-free survival (PFS) was observed with IO102-IO103 plus pembrolizumab versus pembrolizumab alone (19.4 months versus 11.0 months; hazard ratio [HR] 0.77; 95% confidence interval [CI] 0.58–1.00; p=0.0558). The objective response rates were 44.8% and 41.2% in the vaccine and control arms, respectively. “Although the primary endpoint was not met and we have to be cautious, the results are clinically interesting, especially in certain subgroups,” comments Dr Ines Pires da Silva from the Melanoma Institute Australia and The University of Sydney, Australia.
These findings echo an editorial authored by Dr Marco Donia, National Center for Cancer Immune Therapy (CCIT-DK), Herlev, Denmark published yesterday, which emphasises that disruptive progress in the field of cancer vaccines may not come from a single breakthrough but from connecting dots across multiple discoveries.
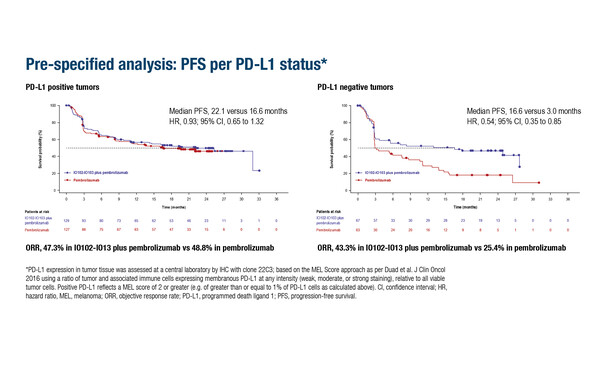
Interesting insights come from a pre-specified analysis of the IOB-013/KN-D18 trial where IO102-IO103 appeared to offer particular benefits to patients with PD-L1-negative tumours (n=130), with a median PFS of 16.6 months in the vaccine arm compared with only 3.0 months with pembrolizumab alone (HR 0.54; 95% CI 0.35–0.85).
“This is actually one of the most interesting aspects of this study,” explains Pires da Silva. “It suggests that the vaccine could help overcome resistance in PD-L1-negative disease and it raises some fascinating biological questions about how these vaccines interact with the immune system.” She is also cautiously optimistic that IO102-IO103 may be beneficial in patients with BRAFV600 mutations or high lactate dehydrogenase (LDH) expression. “Results are encouraging in these subgroups, but they highlight the need for further studies to confirm these findings and to better understand which patients derive the greatest benefit,” she observes. Safety findings were reassuring, with treatment-related serious adverse events occurring at a lower rate in the vaccine arm compared with the control arm (9.5% versus 12.6%).
Pires da Silva concludes: “These results provide valuable insights, particularly regarding patient subgroups that may benefit most from this combination. It is also worth considering that patients who fail to respond to PD-1 inhibitors in the neoadjuvant setting could represent an optimal population for testing the administration of this vaccine in the adjuvant setting. These findings underscore the need for further translational studies to understand the underlying biology and how the vaccine stimulates the immune system.”
Programme details:
Hassel JC, et al. IO102-IO103 cancer vaccine plus pembrolizumab for first-line (1L) advanced melanoma: Primary phase 3 results (IOB-013/KN-D18). ESMO Congress 2025 - LBA53