Blocking GDF-15, an mRNA-based therapy and targeting VISTA have a strong mechanistic rationale, resulting in clinical benefit in heavily pre-treated patients
Over the past decade, immune checkpoint inhibitors (ICIs) have revolutionised the treatment of multiple cancer types, including unresectable metastatic malignancies. However, despite demonstrating durable responses with unsurpassed efficacy in some tumours, patients who initially experience favourable responses can relapse and present with progressive disease due to acquired secondary resistance.
Resistance to ICIs continues to be one of the biggest challenges in cancer care in the face of remarkable immunotherapeutic advances. While ICI rechallenge after initial treatment discontinuation was shown to have a modest benefit in improving patient outcomes in a recent systematic review, achieving an overall objective response rate (ORR) of only 19.4% and a disease control rate of 54.8% (Cancer Med. 2024;13:e70324), other therapeutic strategies hold promise to overcome resistance to ICI therapy and restore sensitivity in patients with anti-PD-(L)1 relapsed/refractory disease.
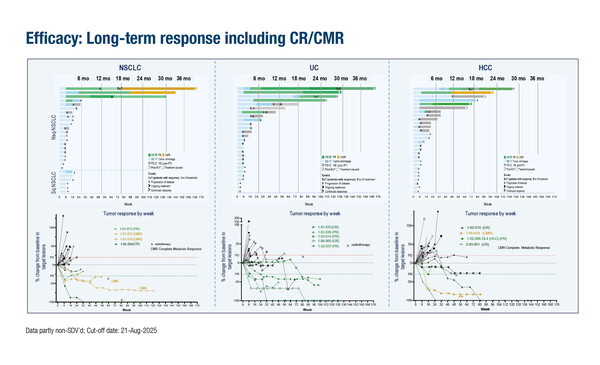
One such strategy is to block growth and differentiation factor 15 (GDF-15), a resistance factor to ICI (Nat Commun. 2023;14:4253), with the neutralising anti-GDF-15 antibody, visugromab. In the first-in-human phase I/IIa GDFATHER-01 trial, patients with advanced cancers refractory to anti-PD-(L)1 therapy showed deep and durable responses following treatment with visugromab in combination with nivolumab (Nature. 2025;637:1218–1227). A long-term follow-up analysis presented at the ESMO Congress 2025 (Berlin, 17–21 October) in 77 heavily pre-treated patients with anti-PD-1/-L1 relapsed/refractory cancer (non-squamous non-small cell lung cancer [NSCLC], urothelial carcinoma [UC] and hepatocellular carcinoma [HCC]) reported confirmed complete responses (CR) or complete metabolic responses (CMR) in 61.5%, 8 of 13 responders (Abstract 1513O). “Notably, response levels were deeper than with their initial, approved first- or second-line ICI treatment regimen,” comments Dr Emanuela Romano from the Center for Cancer Immunotherapy at the Institut Curie in Paris, France.
The median duration of response was 32.2 months, 28.8 months and 19.4 months for NSCLC, UC and HCC, respectively. “The long-term benefit, with 61.5% of responses still ongoing, suggests additional therapeutic options for patients who become refractory to ICIs,” she adds.
Another promising strategy involves an mRNA-based therapy (mRNA-4359) encoding PD-L1 and IDO1 antigens, designed to elicit T-cell responses against tumour and immunosuppressive cells. At ESMO 2025, data were presented from a cohort of patients with ICI relapsed/refractory melanoma treated with mRNA-4359 plus pembrolizumab (Abstract 1515MO). “The patients were very heavily pre-treated, with a median of 3 prior lines of ICI therapy and some patients had received up to 8 prior lines of therapy,” notes Romano. “Despite this, the safety profile seems remarkably good, with mostly grade 1 or 2 adverse events reported.” Also, all responders (6/9, 67%) had a pre-treatment PD-L1 tumour proportion score of ≥1%. “Although we are looking at small subgroups of patients, and the PD-L1 biomarker is known to be suboptimal, it does appear to discriminate well between responders and non-responders to this treatment,” comments Romano.
A different approach consists of targeting V-domain immunoglobulin suppressor of T-cell activation (VISTA), an inhibitory T-cell checkpoint and mediator of immune suppression that is believed to contribute to ICI resistance. Efficacy and safety data for the selective antibody, solnerstotug combined with cemiplimab in patients with advanced solid tumours resistant to prior anti-PD-(L)1 therapy –are encouraging: the ORR was 14% (5/35), with 1 CR and 4 partial responses observed (Abstract 1521MO). “It is quite impressive that some patients experienced responses, as disease stabilisation itself would be considered a good outcome in this population,” she adds, noting that 24% of patients who had received prior anti-PD-(L)1 therapy remain on treatment, with a 6-month PFS rate of 37%. That there were no discontinuations due to treatment-related adverse events is also encouraging given that other anti-VISTA antibodies have been associated with dose-limiting cases of cytokine release syndrome (Nat. Commun. 2024;15:2917). “Preliminary findings suggest this treatment is worth testing further in the clinic,” she says.
Taken together, the presented findings indicate that early-phase research is moving in the right direction to overcome resistance to ICI. “Each of the studies has a strong rationale and the data suggest patient outcomes are better than would be expected with ICI monotherapy rechallenge alone in these heavily pre-treated ICI relapsed/refractory populations, and without major safety concerns. These novel therapeutic strategies may offer an additional opportunity for longer-term disease control,” concludes Romano.
At a glance:
Melero I, et al. Long-term follow-up GDFATHER-01 trial: GDF-15 neutralization combined with nivolumab can enable deep, long-term remission in heavily pretreated, anti-PD1/-L1 relapsed/refractory non-squamous non-small cell lung cancer (NSCLC), urothelial cancer (UC) and hepatocellular cancer (HCC). ESMO Congress 2025 - Abstract 1513O
- ORR: 18.2% in non-squamous NSCLC (n=22); 18.5% in UC (n=27); 14.3% in HCC (n=28)
- CR or CMR: 61.5% overall
- DoR: 32.2 months in non-squamous NSCLC, 28.8 months in UC and 19.4 months in HCC
- Grade ≥3 TRAEs in 10.6% pts
Pinato DJ, et al. Clinical outcomes and PD-L1 expression analyses from a trial of mRNA-4359 plus pembrolizumab in checkpoint inhibitor-resistant/refractory (CPI-R/R) melanoma. ESMO Congress 2025 - Abstract 1515MO
- Among 25 efficacy evaluable patients: ORR: 24%; all responders had pre-treatment PD-L1 TPS ≥1%
- DCR: 60%
- Median DoR: not reached (median follow-up 19.9 weeks)
Papadopoulos KP, et al. Results from a Phase 1 expansion cohort of solnerstotug (pH-selective anti-VISTA antibody) combined with cemiplimab in patients with advanced solid tumors resistant to prior PD-(L)1 therapy. ESMO Congress 2025 - Abstract 1521MO
- ORR: 14% of 35 pts (all with solnerstotug 15 mg/kg + cemiplimab 350 mg)
- CR: 3% (1 pt with Merkel cell carcinoma [MCC])
- PR: 11% (1 pt each with MCC, MSI-H CRC, H&N cancer and oesophageal cancer)
- No discontinuations due to TRAEs
- CRS was rare and low grade (1 TEAE; 2% of all TEAEs)