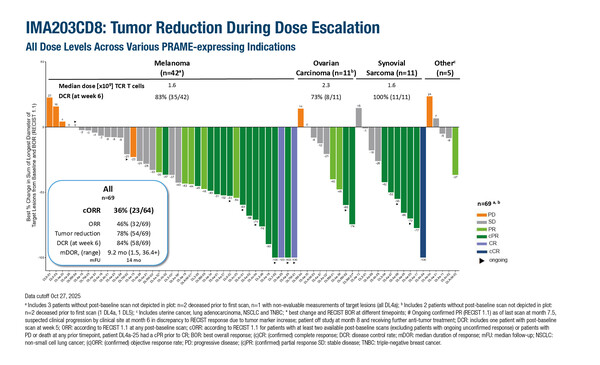
Tumour reduction was observed with IMA203CD8 across different PRAME-expressing cancers in a phase I trial
“PRAME is a very attractive target due to its expression in multiple tumour types and restricted expression in normal tissues (Am J Surg Pathol. 2022;46:1467−1476), and has recently emerged as a promising candidate for T-cell receptor (TCR)-engineered T-cells, which recognise intracellular cancer-associated antigen peptides presented by HLA and initiate a potent and specific immune response,” explains Dr Elena Garralda from Vall d' Hebron Institute of Oncology, Barcelona, Spain, commenting on promising early findings presented at the ESMO Immuno-Oncology Congress 2025 (London, 10–12 December).
In a phase I trial, the next-generation PRAME-directed TCR T-cell therapy, IMA203CD8, showed encouraging activity in melanoma, ovarian carcinoma and synovial sarcoma (Abstract 77MO). Autologous T-cells were transduced with PRAME-targeting TCR and CD8αβ and expanded ex vivo. Patients with HLA-A*02:01-/PRAME-positive recurrent or refractory solid tumours underwent lymphodepletion before receiving a single infusion of IMA203CD8 with or without low-dose interleukin (IL)-2. The objective response rate was 46% among 69 evaluable patients. The disease control rate at 6 weeks was 83% in 42 patients with melanoma, 73% in 11 patients with ovarian cancer and 100% in 11 patients with synovial sarcoma. Overall, as of October 2025, responses were ongoing in 11 patients, with 2 patients to date having experienced confirmed partial responses beyond 2 years.
The most frequent treatment-emergent adverse events were cytopenias associated with lymphodepletion. In total, 74 out of 78 patients evaluable for safety experienced cytokine release syndrome, which was grade 3 in 7 patients and grade 4 in 1 patient. Dose expansion at ~7.2–10 billion total TCR T-cells is ongoing.
These results follow positive news with the PRAME-directed TCR T-cell therapy, IMA203, in uveal melanoma presented at the ESMO Congress 2025 (Ann Oncol. 2025;36(Suppl. 2):S946). Garralda notes the potential advantages of IMA203CD8 over IMA203: “Next-generation IMA203CD8 was designed to achieve enhanced anti-tumour activity via co-transduction of CD8αβ, leading to activation of CD4+ T-cells and CD8+ T-cells. This may produce deeper and more durable responses than with IMA203. While phase I data with IMA203CD8 seem positive, longer follow-up in more patients is needed to confirm this. More translational data on TCR T-cell expansion and persistence would also be valuable.”
Garralda thinks that the augmented action of IMA203CD8 may also be responsible for the broad tumour efficacy. “Uveal melanoma has high PRAME expression but here we see responses in cancers with lower PRAME expression – ovarian cancer and synovial sarcoma – and I look forward to seeing detailed dose-expansion data in these and other tumour types.” Whether the low-dose IL-2 regimen is adding any meaningful benefit is another important area for investigation. The next logical step is to combine IMA203CD8 with other immunotherapies such as immune checkpoint inhibitors, according to Garralda, who concludes: “These types of TCR T-cell therapies are for patients who are positive for HLA-A*02:01 and it would be interesting to see this type of research for other HLAs.”
Programme details:
Busse A, et al. A phase I trial of IMA203CD8, a PRAME-directed TCR T cell therapy in PRAME-positive solid tumors. ESMO Immuno-Oncology Congress 2025 - Abstract 77MO