Additional exploratory analyses of the phase III CheckMate 816 trial reveal that event-free survival at 3 years is not influenced by surgical parameters and suggest that tumour inflammation may be a useful predictive biomarker
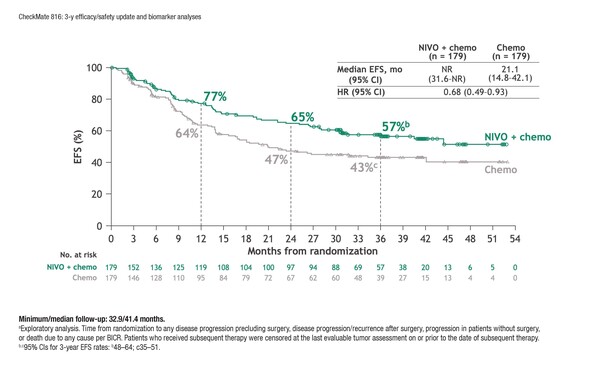
According to results presented at the European Lung Cancer Congress 2023 (Copenhagen, 29 March–1 April), long-term analysis of the phase III CheckMate 816 trial (at a median follow-up of 41.4 months) demonstrated that neoadjuvant nivolumab plus platinum doublet chemotherapy prolonged event-free survival (EFS) compared with chemotherapy alone (Abstract 84O). The findings were observed among 358 patients with stage IB–IIIA resectable NSCLC and no known EGFR/ALK alterations (hazard ratio [HR] 0.68; 95% confidence interval 0.49–0.93) and extend those of the initial trial analysis conducted at a minimum follow-up of 29.5 months (N Engl J Med. 2022;386:1973–1985). Grade 3–4 treatment-related and surgery-related adverse events occurred at similar rates in each arm.
Exploratory analyses showed that 3-year EFS rates were higher with nivolumab plus chemotherapy versus chemotherapy, both among patients treated with minimally invasive surgery (67% versus 53%) and among those receiving thoracotomy or conversion (61% versus 51%). Three-year EFS rate improvements with nivolumab plus chemotherapy versus chemotherapy alone were also not dependent on the extent of resection (with rates of 64% versus 49% for lobectomy and 67% versus 48% for pneumonectomy) and were evident in patients with complete (R0) resection (with rates of 64% versus 51%). Among patients undergoing surgery, recurrence rates were 28% in those receiving nivolumab plus chemotherapy and 42% in those receiving chemotherapy alone.
In patients receiving nivolumab plus chemotherapy, a baseline 4-gene inflammatory signature score was numerically higher in those achieving a pathological complete response. In addition, a high inflammatory score was linked to an improved EFS.
“These maturing data are very important and represent a big step forward in curing patients with resectable NSCLC,” says Prof. Martin Reck from Lung Clinic Grosshansdorf, Germany. “Because EFS is considered a surrogate marker for overall survival, the probability that the results from the CheckMate 816 trial will transition into a real survival benefit is increasing.” Another important point is that the benefit was seen across all patients, independent of the type of surgery and the surgical access to the lung. “However, overall survival results will be necessary to determine the true value of this approach,” cautions Reck. “In addition, we need to consider the suggestion from the original analysis that EFS benefit was driven by patients achieving a pathological complete response.”
Based on the results from the CheckMate 816 trial, Reck thinks it will not be long before clinical practice is changed and this will hasten the search for predictive biomarkers to identify patients who are the best candidates for neoadjuvant chemoimmunotherapy. He also calls for a redefinition of resectability: “Until recently, nodal involvement of the mediastinum was a clear criterion against resectability, but we are now operating on node-positive tumours following neoadjuvant chemoimmunotherapy. Perioperative chemoimmunotherapies are dramatically changing the face of non-resectable, locally advanced NSCLC and will hopefully enable resections in a greater number of patients.”
Concluding, Reck comments: “An important issue for clinical practice will be to differentiate the results seen with pure neoadjuvant chemoimmunotherapy, as used in CheckMate 816, from those soon to be reported from the new generation of phase III trials investigating neoadjuvant plus adjuvant chemoimmunotherapy in resectable NSCLC.”
Abstract discussed:
Girard N, et al. Neoadjuvant nivolumab (N) + platinum-doublet chemotherapy (C) for resectable NSCLC: 3-y update from CheckMate 816. European Lung Cancer Congress 2023, Abstract 84O
Proffered Paper 2, 30.03.2023, h. 15:10 – 16:40, Auditorium 1