Updated results from the CHRYSALIS trial also suggest a number of biomarkers predictive of sustained clinical benefit with the EGFR–MET bispecific antibody
According to a presentation at the European Lung Cancer Congress 2023 (Copenhagen, 29 March–1 April), the bispecific EGFR-MET monoclonal antibody, amivantamab led to a median overall survival of 23 months (95% confidence interval [CI] 18.5–29.5) among 114 patients with non-smallcell lung cancer (NSCLC) with EGFR Exon 20 insertion mutations after progression on platinum-based chemotherapy (Abstract 3O). At a median follow-up of 19.2 months, the overall response rate (ORR) was 37% (95% CI 28–46), median duration of response was 12.5 months (95% CI 6.9–19.3) and median progression-free survival was 6.9 months (95% CI 5.6–8.8). Rash (89%) and infusion-related reactions (67%) were the most frequently reported all-grade toxicities.
Amivantamab showed consistent activity across subgroups, including type of prior therapy (ORRs of 42.0% for prior immunotherapy and 52.2% for prior EGFR tyrosine kinase inhibitor therapy) and response to prior platinum-based chemotherapy (ORRs of 36.2% for patients with response or stable disease and 31.2% of those with disease progression).
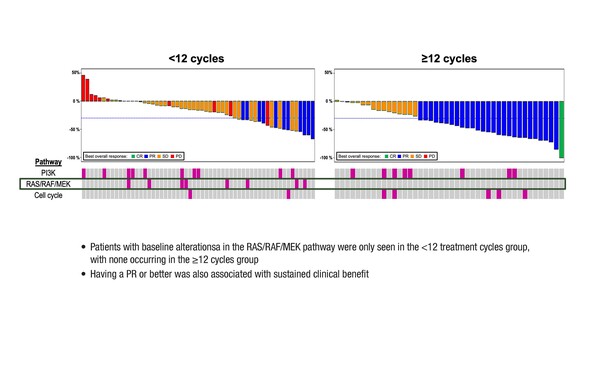
Forty-eight patients (42%) had sustained clinical benefit with amivantamab (defined as receipt of ≥12 cycles) and this was associated with an ECOG performance status of 0, achieving at least a partial response and not having baseline alterations in the RAS/RAF/MEK pathway. Among 15 patients continuing to receive amivantamab after a median of 2.6 years, seven remain progression-free.
“It is reassuring to see that the mature efficacy data in this analysis are generally consistent with the initial findings, for both the overall analysis and the subset analyses (J Clin Oncol. 2021;39:3391–3402),” says Dr Ross Soo from the National University Cancer Institute Singapore. “The data show a relatively high rate of infusion-related reactions, but this is in line with what is expected with amivantamab and is the same frequency as that reported in the original analysis.” He notes that the indicators of clinical benefit are particularly promising, given the importance of tailoring treatment to avoid patients who are unlikely to respond.
According to Soo, EGFR Exon 20 insertion-mutated NSCLC is attracting a lot of drug development activity. “Currently, amivantamab is only one of two agents with regulatory approval for pretreated disease, with mobocertinib the other agent that is approved in some countries in this setting. However, there are many other agents in development. Such agents include zipalertinib (CLN-081/TAS6417), sunvozertinib, furmonertinib and, at an earlier stage of development, BLU-451 and ORIC-114. It will be especially interesting to see results with small-molecule inhibitors that have CNS-penetrating activity, which is limited with amivantamab and mobocertinib. We also need to look at how we can use these types of inhibitors in patients with untreated disease.” The phase III PAPILLON study is investigating amivantamab plus chemotherapy versus chemotherapy in the frontline EGFR Exon 20 insertion-mutated NSCLC setting (NCT04538664).
Abstract discussed:
Garrido Lopez P, et al. Long-term efficacy, safety, and predictors of response to amivantamab among patients with post-platinum EGFR Ex20ins-mutated advanced NSCLC. European Lung Cancer Congress 2023, Abstract 3O
Proffered Paper 1, 29.03.2023, h. 16:30 – 18:00, Auditorium 1