Data from the TALENTACE trial add to growing evidence supporting the combination of immunotherapy plus anti-VEGF component with TACE
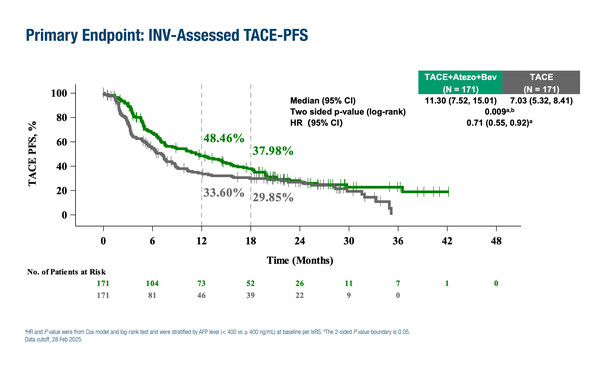
According to results presented at the ESMO Gastrointestinal Cancers Congress 2025 (Barcelona, 2–5 July), atezolizumab plus bevacizumab after on-demand transarterial chemoembolisation (TACE) significantly improved the primary endpoint of investigator-assessed TACE-progression-free survival (PFS) compared with TACE alone (median 11.30 months versus 7.03 months; hazard ratio [HR] 0.71; 95% confidence interval [CI] 0.55–0.92; p=0.009) in systemically untreated, intermediate-to-high tumour burden, unresectable hepatocellular carcinoma (HCC) (LBA2). The phase III TALENTACE trial involved 342 patients with TACE-eligible untreated HCC and a sum of tumour maximum diameter plus tumour number ≥6. TACE-PFS was defined as the time from randomisation to untreatable progression or TACE failure/refractoriness or death by any cause. The median follow-up was around 26 months.
The benefit of the TACE–atezolizumab/bevacizumab combination was also evident in PFS assessed per RECIST v1.1 (median 10.32 months versus 6.37 months; HR 0.64; 95% CI 0.50–0.82). At the first interim analysis, median overall survival (OS) was 34.53 months with TACE–atezolizumab/bevacizumab versus 35.38 months with TACE alone (HR 0.96; 95% CI 0.68–1.34). The TACE–atezolizumab/bevacizumab arm demonstrated a higher overall response rate compared to the TACE alone arm according to RECIST v1.1 (49.1% versus 33.9%) and RECICL (81.3% versus 66.7%).
Grade 3–4 treatment-related adverse events (TRAEs) occurred in 60.8% of patients receiving TACE–atezolizumab/bevacizumab compared with 40.5% receiving TACE alone. Serious TRAEs were seen in 25.9% of patients receiving TACE–atezolizumab/bevacizumab and 13.9% receiving TACE alone. There were five treatment-related deaths in the TACE–atezolizumab/bevacizumab arm and three in the TACE-alone arm.
Commenting on the findings, Prof. Anna Saborowski from Hannover Medical School, Germany, remarks: “These positive findings add to those from the recent placebo-controlled EMERALD-1 and LEAP-012 phase III trials, which demonstrated significant PFS improvement with TACE plus durvalumab/bevacizumab and TACE plus pembrolizumab/lenvatinib, respectively, albeit with design differences related to tumour burden, and the number and timing of TACE (Lancet. 2025;405:216–232; Lancet. 2025;405:203–215).”
Saborowski thinks that mature OS data – which have also not been published for the EMERALD-1 or LEAP-012 trials – will be pivotal in determining the place of TACE combination therapy: “If there is no prolongation of OS, then we may have to consider how important improved PFS is as an endpoint and use patient-reported outcomes as a way of assessing benefit in the face of treatment-related adverse events.”
Other novel strategies being investigated in intermediate-stage HCC include the EMERALD-3 trial assessing Single Tremelimumab Regular Interval Durvalumab (STRIDE) with or without lenvatinib plus TACE (NCT05301842), the ABC-HCC trial comparing atezolizumab/bevacizumab versus TACE (NCT04803994) and the EMERALD-Y90 trial evaluating transarterial radioembolisation plus durvalumab/bevacizumab (NCT06040099).
Programme details
Dong J, et al. TALENTACE: A phase III, open-label, randomized study of on-demand transarterial chemoembolization (TACE) combined with atezolizumab + bevacizumab (Atezo+Bev) or on-demand TACE alone in patients with systemically untreated, intermediate-to-high burden unresectable hepatocellular carcinoma (uHCC). ESMO Gastrointestinal Cancers Congress 2025, LBA2
Proffered Paper Session 1, 03.07.2025, h. 14:00 – 15:30, Room Barcelona