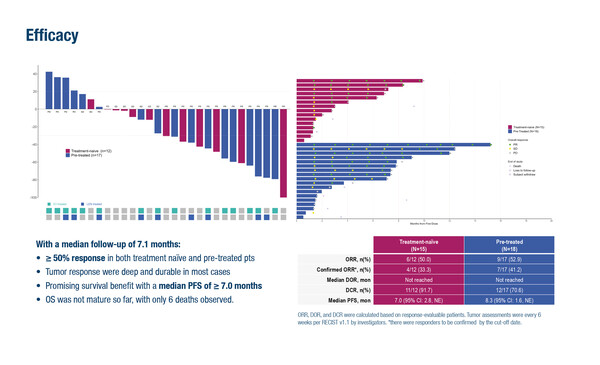
Encouraging data were presented for FGF19-positive tumours and those with worse hepatic function, performance status or advanced vascular invasion
At the ESMO Gastrointestinal Cancers Congress 2025 (Barcelona, 2–5 July), updated results from the phase II ABSK-011-201 study demonstrated that irpagratinib, a fibroblast growth factor receptor-4 (FGFR4) inhibitor, plus atezolizumab produced an overall objective response rate of 51.7% in 29 evaluable patients with advanced hepatocellular carcinoma (HCC) and FGF19 overexpression (Abstract 149MO).
At a median follow-up of 7.1 months, the median duration of response (DOR) had not been reached with the combination therapy yet. Most responders were still ongoing at the time of analysis. Responses were seen in both pre-treated and first-line subgroups (52.9% and 50.0%, respectively). Median progression-free survival (PFS) was ≥7.0 months and overall survival was not yet evaluable.
“These are exciting data for patients with FGF19-positive tumours, which are associated with a poor prognosis (BMC Cancer. 2012;12:56),” says Dr Rachna Shroff from the University of Arizona Cancer Center, Tucson, AZ, USA. “Both pre-treated and untreated patients experienced a response rate in the order of 50% and we have not reached the median DOR yet. However, additional details about the selection criteria, including how patients were screened for FGF19, would be of interest.” The study was conducted in China and ~85% of patients were positive for hepatitis B virus (HBV). “This is a very specific, Eastern population and we need to better understand the translatability to more international populations without HBV-associated cirrhosis,” Shroff points out.
In terms of safety, elevated liver function tests were among the most common treatment-related adverse events (TRAEs), but the incidence of grade 3 events was low: alanine aminotransferase (ALT) increase in 12.1% and aspartate aminotransferase (AST) increase in 9.1%. There were no grade 4 or 5 TRAEs and, importantly, no patients discontinued treatment due to TRAEs.
A second study presented at the Congress provides some encouraging data for the use of the Single Tremelimumab Regular Interval Durvalumab (STRIDE) regimen as first-line treatment for patients with HCC and a poor prognosis (Abstract 150MO). No unexpected concerns were reported in a safety analysis of the phase IIIb SIERRA trial, which included patients with worse hepatic function, poorer Eastern Cooperative Oncology Group performance status (ECOG PS) or more advanced vascular invasion than the HIMALAYA trial from which approval of the STRIDE regimen was obtained (NEJM Evid. 2022;1:EVIDoa2100070). Participants in the SIERRA trial had a Child-Pugh (CP) score of: B7 or B8 with an ECOG PS of 0–1 (n=35); a CP score of A with an ECOG PS of 2 (n=44); or CP score of A, ECOG PS 0–1 and chronic main trunk portal vein thrombosis (n=19). Grade 3 or 4 adverse events (AEs) occurring within 6 months considered to be possibly related to study treatment were reported in 19.4% of patients, while serious AEs possibly related to study treatment occurred in 14.3% of patients. AEs led to treatment discontinuation in 10.2% of patients.
“The study is really important because these are the patients we see every day in the clinic,” explains Shroff. Although the patient subsets are small, she notes: “It should be possible to expand the patient population eligible for this treatment; however, it will first be important to assess efficacy in this broader population when results become available.”
Programme details
Cheng Q, et al. Irpagratinib (ABSK-011) plus atezolizumab in first-line (1L) and immune checkpoint inhibitors (ICIs) treated advanced hepatocellular carcinoma (HCC) with FGF19 overexpression (+): Updated results of the phase 2 ABSK-011-201 study. ESMO Gastrointestinal Cancers Congress 2025, Abstract 149MO
Mini Oral Session – Innovation in GI cancers, 02.07.2025, h. 16:15 – 17:35, Room Barcelona
Chan SL, et al. Safety results from the phase 3b SIERRA study of durvalumab (D) and tremelimumab (T) as first-line (1L) treatment (tx) for hepatocellular carcinoma (HCC) participants (pts) with a poor prognosis. ESMO Gastrointestinal Cancers Congress 2025, Abstract 150MO
Mini Oral Session – Innovation in GI cancers, 02.07.2025, h. 16:15 – 17:35, Room Barcelona