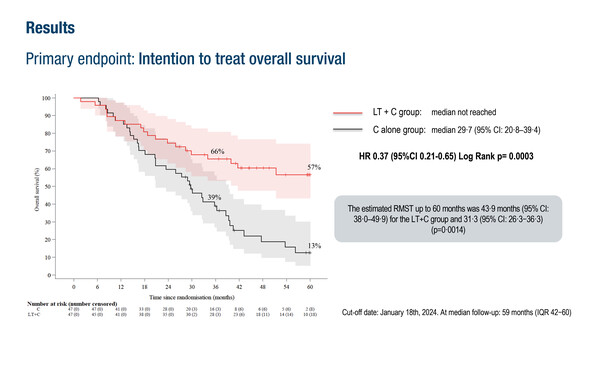
In the TRANSMET trial, a 5-year overall survival benefit was observed in a highly selected patient population
Updated results from the randomised TRANSMET trial showed that chemotherapy combined with liver transplantation in highly selected patients with colorectal cancer quadrupled the 5-year overall survival (OS) rate compared with chemotherapy alone (Abstract 1O). Data were presented at the ESMO Gastrointestinal Cancers Congress 2024 (Munich, 26–29 June), and anticipated at the 2024 ASCO Annual Meeting (J Clin Oncol. 2024;42(Suppl 16):3500).
“The 5-year OS data are extremely good, and beyond what was expected,” says Prof. Volker Heinemann from the Munich Comprehensive Cancer Center, Munich, Germany, commenting on the study results. Apart from non-resectability evaluated by an independent expert committee, the study inclusion criteria required that patients had resection of the primary tumour, metastases strictly confined to the liver, BRAF wild-type disease and response to up to three lines of chemotherapy (lasting ≥3 months).
In an intention-to-treat (ITT) analysis of 94 patients, the 5-year OS rate was 57% in the chemotherapy plus liver transplantation arm and 13% with chemotherapy alone (hazard ratio [HR] 0.37; 95% confidence interval [CI] 0.21–0.65; p=0.0003). In the per-protocol analysis of 74 patients, corresponding 5-year OS rates were 73% versus 9%, respectively (HR 0.16; 95% CI 0.07–0.33; p<0.0001) were reported. Median progression-free survival was 17.4 months in the chemotherapy plus liver transplantation arm and 6.4 months with chemotherapy alone (HR 0.34; 95% CI 0.20–0.58; p<0.0001). Twenty-eight (74%) patients who underwent the combination treatment had recurrence, with 13 (46%) optionally treated by surgery or local ablation. Altogether, 15 patients in this treatment arm were ultimately disease free.
“The data on recurrence are particularly interesting,” Heinemann continues. “The markedly longer survival in the chemotherapy plus liver transplantation arm was achieved despite the fact that three-quarters had disease recurrence. This highlights that the liver is the most important metastatic site with regard to OS and that removal of liver metastases may provide a tremendous survival benefit, irrespective of cure.”
Heinemann now questions what these findings will mean for clinical practice: “These data will undoubtedly change the way we look at treating some patients with unresectable liver metastases. However, this was a very selected patient group and the requirement to meet the selection criteria – including the absence of extrahepatic disease, which becomes increasingly common with successive lines of therapy – means that only a very small fraction of patients will be eligible for this approach.” In addition, logistical considerations include the need to put in place expert panels required for adequate patient selection and to generate access to centres focussing on liver transplantation. Other challenges that need to be addressed are the scarcity of donor organs and prioritisation of liver transplantation in patients with cancer (BJS Open. 2020;4:467–477). “Expanding the criteria for living donor liver transplantation could be considered, but this is associated with its own ethical issues,” he concludes.
Abstract discussed:
Adam R, et al. Chemotherapy and liver transplantation versus chemotherapy alone in patients with definitively unresectable colorectal liver metastases: Updated results from the randomized TRANSMET trial. ESMO Gastrointestinal Cancers Congress 2024, Abstract 1O
Proffered Paper Session, 27.06.2024, h. 14:00 – 15:35, Room 14