Final results of the KEYNOTE-585 study indicate that the current treatment standard for resectable G/GEJ should remain unchanged
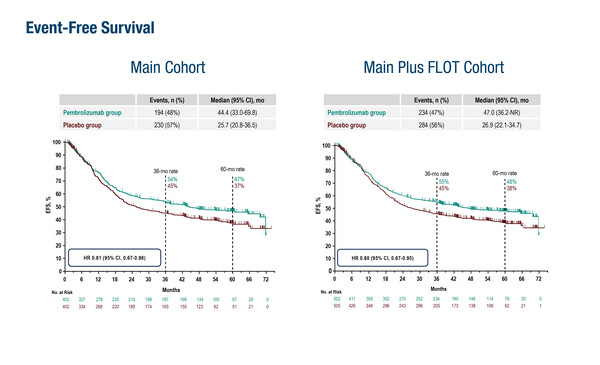
As presented at the ESMO Gastrointestinal Cancers Congress 2024 (Munich, 26–29 June), final analysis of the KEYNOTE-585 study showed that event-free survival was not significantly improved with pembrolizumab plus CT compared with placebo plus CT or placebo plus FLOT in patients with untreated, locally advanced, resectable gastric/gastro-oesophageal junction (G/GEJ) cancers (LBA3). At a median follow-up of 59.9 months, neoadjuvant plus adjuvant treatment was associated with a median event-free survival (EFS) of 44.4 months with pembrolizumab plus cisplatin/fluorouracil-based chemotherapy (CT) versus 25.5 months with placebo plus CT (hazard ratio [HR] 0.81; 95% confidence interval [CI] 0.67–0.98) among the 804 patients involved in the study. Median overall survival (OS) was 71.8 months with pembrolizumab versus 55.7 months with placebo (HR 0.86; 95% CI 0.71–1.06). The pathological complete response (pCR) rates were 13.4% with pembrolizumab and 2.0% with placebo.
Results were similar when an additional 203 patients were incorporated from a separate cohort who received FLOT (docetaxel, oxaliplatin, leucovorin and 5-fluorouracil) instead of cisplatin/fluorouracil CT. Median EFS was 47.0 months versus 26.9 months (HR 0.80; 95% CI 0.67–0.95), median OS was 71.8 months versus 55.7 months (HR 0.86; 95% CI 0.71–1.06) and pCR rates were 14.2% and 2.8%, with pembrolizumab versus placebo, respectively.
“The numerical increases seen in EFS and OS in this final analysis are consistent with previously reported interim results (Lancet Oncol. 2024;25:212–224), but they are not statistically significant and so are not sufficient to change our current standard practice of perioperative FLOT for resectable G/GEJ cancers,” comments Dr Elizabeth Smyth from the Oxford University Hospitals NHS Foundation Trust, UK. “However,” she says, “the increase in the pCR rate seen with the addition of pembrolizumab suggests it may improve response to CT in at least some patients. It will be interesting to explore the efficacy of the PD-1 inhibitor plus CT combination in those cancers that we know are particularly sensitive to immune checkpoint inhibitors, such as tumours that have high microsatellite instability and/or PD-L1 expression.” Smyth continues: “Next steps will be, to some extent, informed by the results of the ongoing MATTERHORN trial – investigating the addition of durvalumab to perioperative FLOT in resectable G/GEJ cancers – which is due to report in 2025.”
Smyth concludes: “A key issue is finding ways to bring effective novel treatments to patients more quickly. The development of better surrogate endpoints for perioperative trials should help to avoid prolonged studies and we need to better understand how pCR, ctDNA, radiomics and digital pathology might help to more accurately predict long-term outcomes. Translational work from KEYNOTE-585 and MATTERHORN may be instrumental in helping to answer these questions.”
Abstract discussed:
Shitara K, et al. Final analysis of the phase 3 KEYNOTE-585 study of pembrolizumab plus chemotherapy vs chemotherapy as perioperative therapy in locally-advanced gastric and gastroesophageal junction cancer. ESMO Gastrointestinal Cancers Congress 2024, LBA3
Proffered Paper Session, 27.06.2024, h. 14:00 – 15:35, Room 14