Innovative approaches are investigated to predict cancer development in patients with cirrhosis and detect early recurrence after resection
While there has been expansion in drug development for the treatment of hepatocellular carcinoma (HCC) over the last decade, the cancer lags behind others in having specific biomarkers to guide management. Studies presented at the ESMO Gastrointestinal Cancers Congress 2024 (Munich, 26–29 June) reveal how advances in technology may improve patient care.
“Predicting which patients with cirrhosis of the liver will develop HCC is a key challenge,” explains Dr David J Pinato from Imperial College London, UK. “High-risk patients with cirrhosis are invited for regular ultrasound and alpha-fetoprotein (aFP) testing but the capacity of these tests to predict cancer is fairly limited. In addition, patients often miss screening appointments because of anxiety around ultrasound tests, and this is compounded by the fact that many live in difficult socioeconomic circumstances,” he says.
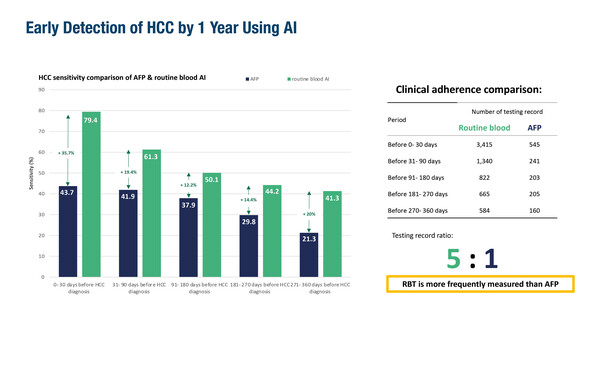
A retrospective study from Hong Kong, presented at the Congress, reported that artificial intelligence (AI) analysis of routine blood tests revealed HCC signatures up to 1 year before the clinical diagnosis of cancer (Abstract 165MO). Using results (including complete blood counts, liver and renal function tests and clotting profiles) from 3,415 patients with HCC showed that AI-associated screening sensitivity was 61.3% at 1–3 months, 50.1% at 3–6 months, 44.2% at 6–9 months and 41.3% at 9–12 months before diagnosis. AI sensitivity was superior to aFP testing, which remained under 45% at all intervals.
“Currently, interpretation of what degree of ‘abnormal’ is a sign of potential HCC development is complex in blood tests from patients with cirrhosis, as results routinely fall outside the normal range,” notes Pinato, who continues, “The capacity of the AI model to define this significance with good specificity is remarkable.” Given that this is a study from Asia, where HCC may have a different aetiology compared with Western countries, leads Pinato to suggest that large-cohort confirmatory studies are required to give a better insight into the global utility of this AI model.
In another retrospective study presented at the Congress, serial ctDNA testing was shown to be effective in identifying early recurrence in patients with HCC undergoing mainly curative-intent surgery (Abstract 181P). Among 64 patients who underwent liver transplantation and subsequent recurrence monitoring, all 36 patients with testing available in the minimal residual disease (MRD) window of up to 12 weeks post-surgery were ctDNA negative and 35 remained negative at a median follow-up of 21.2 months. Of 61 patients with imaging available within 6 months of a ctDNA test, 99.5% sample level specificity was observed. Among 47 patients who underwent hepatectomy and subsequent recurrence monitoring, ctDNA was detected in 7 of 31 patients (22.5%) within the MRD window; 4 experienced clinical recurrence and 3 had a short follow-up. Of 42 patients with imaging available within 6 months of testing, ctDNA positivity was prognostic for recurrence, with a hazard ratio for recurrence-free survival of 12 (p<0.0001). ctDNA also showed utility in monitoring treatment response in patients with known recurrence.
“The ability to use dynamic tools, such as ctDNA, could boost our capacity to detect patients who are inevitably going to relapse and could be useful in more accurately tailoring treatment, for example, selecting who receives adjuvant therapy to help improve outcomes,” concludes Pinato.
Abstracts discussed:
Kwok KN, et al. Early detection of HCC by routine blood-based AI. ESMO Gastrointestinal Cancers Congress 2024, Abstract 165MO
Mini Oral Session 1, 26.06.2024, h. 16:30 – 18:00, Room 14
Abdelrahim M, et al. Circulating tumor DNA (ctDNA) testing for recurrence and treatment response monitoring in hepatocellular carcinoma (HCC). ESMO Gastrointestinal Cancers Congress 2024, Abstract 181P
Poster Display Session, 27.06.2024, h. 15:35 – 16:30, Foyer