Promising data from a subgroup analysis of the phase III NETTER-2 study support the use of peptide receptor radionuclide therapy in treatment-naïve patients
Peptide receptor radionuclide therapy (PRRT) with [177Lu]Lu-DOTA-TATE (177Lu-DOTATATE) has demonstrated efficacy across grade 2 high-grade 3 low gastroenteropancreatic neuroendocrine tumours (GEP NETs) (Lancet. 2024: S0140-6736(24)00701-3), including tumours of different primary origins in a subgroup analysis of the phase III NETTER-2 study presented at the ESMO Gastrointestinal Cancers Congress 2024 (Munich, 26–29 June) (Abstract 211MO).
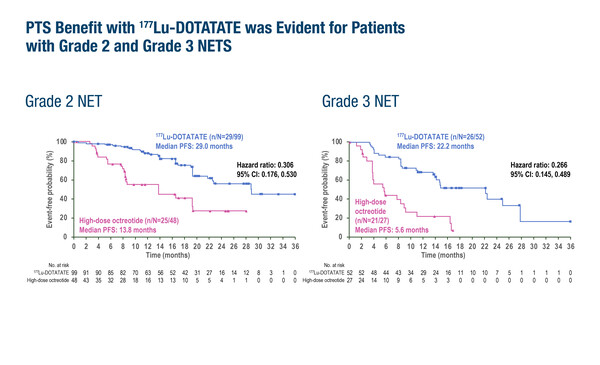
In the pre-planned subgroup analysis, the median progression-free survival (mPFS) was longer for the 177Lu-DOTATATE arm versus the high-dose octreotide control arm, regardless of tumour grade (grade 2: 29.0 months versus 13.8 months; grade 3: 22.2 months versus 5.6 months) or primary tumour origin (pancreas: 19.4 months versus 8.5 months; small intestine: 29.0 versus 8.4 months). Objective response rates (ORRs) were also notably higher with 177Lu-DOTATATE compared with control across all subgroups.
Currently, first-line therapy for higher grade GEP-NETs consists of cytotoxic chemotherapy, although evidence to support this common practice is rather weak for primary sites other than the pancreas, highlighting a significant unmet need for these patients. Moreover, grade 3 GEP-NETs are a relatively recently defined category of NETs and data on optimal treatment strategies in this setting are lacking. No therapies have been specifically approved for the treatment of grade 3 GEP-NETs and current guidelines recommend treatments based on those for rapidly progressing grade 2 high disease.
Commenting on the subgroup analysis findings, Dr Rocio Garcia-Carbonero from Hospital Universitario Doce de Octubre, Madrid, Spain, notes, “This is a critical study as it is the first randomised controlled trial to assess PRRT and provide specific efficacy data for grade 3 NETs and for treatment-naïve patients.”
The randomised controlled NETTER-2 trial evaluated the efficacy of 177Lu-DOTATATE as first-line treatment in newly diagnosed patients with grade 2 high or grade 3 low (Ki67 10%–55%) well-differentiated GEP-NETs. The primary results, presented earlier this year (J Clin Oncol. 2024;42(3_suppl):LBA588), demonstrated a significantly improved mPFS and increased ORR in patients treated with 177Lu-DOTATATE (n=151) compared with those receiving control (n=75).
Regarding the use of this treatment in the clinic, Garcia-Carbonero remarks, “The activity shown by 177Lu-DOTATATE in the NETTER-2 study is highly clinically relevant and supports its use as a frontline treatment for somatostatin receptor-positive grade 3 low GEP-NETs and maybe selected patients with grade 2 high GEP NETs.” However, she cautions that the NETTER-2 trial does not compare the effectiveness of 177Lu-DOTATATE to the recommended standard treatment for patients with grade 2 or grade 3 GEP-NETs (everolimus, sunitinib or chemotherapy) – a question that ongoing head-to-head studies aim to address. Additionally, there is a need to better understand toxicities associated with 177Lu-DOTATATE and how they may impair further treatments down the line before it can be recommended as the frontline treatment of choice for all patients with grade 2 high-grade 3 low GEP-NETs.
Abstract discussed:
Singh S, et al. First-Line Efficacy of [177Lu]Lu-DOTA-TATE in Patients with Advanced Grade 2 and Grade 3, Well-Differentiated Gastroenteropancreatic Neuroendocrine Tumors by Tumor Grade and Primary Origin: Subgroup Analysis of the Phase 3 NETTER-2 Study. ESMO Gastrointestinal Cancers Congress 2024, Abstract 211MO
Mini Oral Session 1, 26.06.2024, h. 16:30 – 18:00, Room 14