Enzalutamide failed to deliver in high-risk localised prostate cancer, but improved survival, in combination with leuprolide, in biochemically recurrent prostate cancer
Adding enzalutamide to standard adjuvant androgen deprivation therapy (ADT) did not improve the primary endpoint of metastasis-free survival (MFS) in the phase III ENZARAD (ANZUP 1303) trial conducted in 802 men undergoing radiotherapy for high-risk localised and locally advanced prostate cancer, according to a Late-Breaking presentation at the ESMO Congress 2025 (Berlin, 17–21 October) (LBA86). MFS at 8 years was 74% in the group that received enzalutamide for 24 months versus 72% in the group that received conventional non-steroidal anti-androgens (NSAA) for 6 months (hazard ratio [HR] 0.88; 95% confidence interval [CI] 0.67–1.15; p=0.34). Eight-year overall survival (OS) was 83% versus 80% with enzalutamide versus NSAA, respectively (HR 0.87; 95% CI 0.63–1.20; p=0.40).
“The results from this trial are rather disappointing and not what I would have expected, given the results of the STAMPEDE trial, where abiraterone plus prednisolone significantly improved OS when given with ADT and radiotherapy (N Engl J Med. 2017;377:338–351),” comments Dr Alison Tree from The Institute of Cancer Research and The Royal Marsden National Health Service Foundation Trust, London, UK.
Despite the high-risk population, she notes that prostate cancer-specific survival was encouragingly high in both groups (97% with enzalutamide and 96% with NSAA) in the ENZARAD trial, potentially highlighting the effectiveness of modern radiotherapy techniques in combination with ADT. Benefits were observed for prostate-specific antigen (PSA) progression-free survival (PFS; HR 0.78; 95% CI 0.61–0.99), but Tree thinks that this may not be sufficient to justify a relatively expensive treatment in all patients. In the ENZARAD trial, 89% of patients had a Gleason score 8–10; 47% had stage T3–4 disease and 35% had PSA ≥20. “It appears that patients may have had a better risk profile than those in STAMPEDE, which may explain why the addition of enzalutamide did not translate into a survival benefit,” notes Tree. The effects of enzalutamide on MFS were larger in the N1 versus N0 subgroup (p for interaction=0.04) and the planned pelvic versus no pelvic radiotherapy subgroup (p for interaction <0.001), which warrants further investigation, according to Tree: “Men with more advanced cancer and those undergoing more extensive radiotherapy appeared to have more scope to gain from enzalutamide.” She notes that results of the ATLAS trial investigating ADT with or without apalutamide in patients with high-risk localised or locally advanced prostate cancer receiving primary radiation therapy (NCT02531516) are now even more eagerly awaited to determine if second-generation anti-androgens can have an impact.
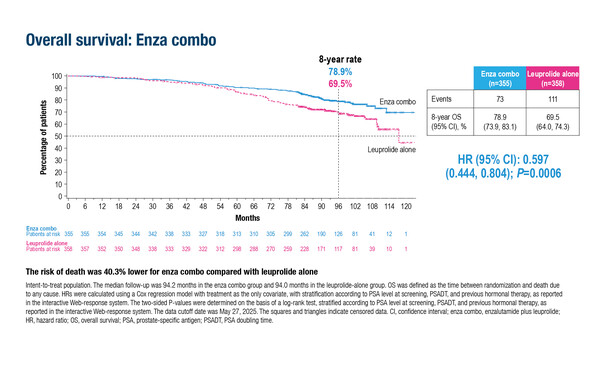
Also presented at the ESMO Congress were positive final OS data from EMBARK (LBA87), which confirmed the significant improvements in MFS with enzalutamide plus leuprolide observed previously in biochemically recurrent prostate cancer (N Engl J Med. 2023;389:1453–1465). In the new analysis in over 1,000 men, enzalutamide plus leuprolide reduced the risk of death by 40.3% compared with leuprolide alone (HR 0.597; 95% CI 0.444–0.804; p=0.0006) after median follow-up of 94 months. The effect of enzalutamide monotherapy versus leuprolide alone on OS did not reach statistical significance (HR 0.830; 95% CI 0.630–1.095; p=0.1867).
Tree notes, “Improving survival from 69.5% with leuprolide alone to 78.9% gives us confidence in the findings of EMBARK, but the treatment landscape for biochemically recurrent prostate cancer has changed considerably in recent years. The current standard of care for biochemical recurrence after surgery alone is prostate bed irradiation, which was not mandated in EMBARK. Importantly, prostate-specific membrane antigen (PSMA)-positron emission tomography (PET) is now used in many centres to stage patients, rather than conventional imaging (computed tomography and bone scan), which can under-detect metastatic disease.” In a recent analysis in a cohort of patients with high-risk, hormone-sensitive prostate cancer without evidence of metastatic disease by conventional imaging, PSMA-PET results were positive in 84% of patients, PSMA-PET detected M1 disease stage in 46% of patients and found polymetastatic disease (≥5 lesions) in 24% of patients (JAMA Netw Open. 2025;8:e2452971).
“I do not think we can necessarily extrapolate the results of EMBARK to today’s PSMA-PET-negative patients, who have a more favourable risk profile than those treated in the trial. With widespread adoption of next-generation imaging, the time is now right to incorporate PSMA-PET into patient selection in trials and clinical settings,” she concludes.
Programme details:
Nguyen PL, et al. Randomised phase III trial of androgen deprivation therapy (ADT) with radiation therapy with or without enzalutamide for high risk, clinically localised prostate cancer: ENZARAD (ANZUP 1303). ESMO Congress 2025 - LBA86
Shore ND, et al. Overall survival with enzalutamide in biochemically recurrent prostate cancer. ESMO Congress 2025 - LBA87