In KEYNOTE-905/EV-303, the combination of an antibody–drug conjugate and immunotherapy showed statistically significant and clinically meaningful improvements in event-free survival and overall survival
The combination of enfortumab vedotin (EV) – an antibody–drug conjugate – and pembrolizumab, before and after standard radical cystectomy and pelvic lymph node dissection, may be a potential new standard of care for cisplatin-ineligible patients with muscle-invasive bladder cancer (MIBC), according to findings presented in today’s Presidential Symposium at the ESMO Congress 2025 (Berlin, 17–21 October) (LBA2). “KEYNOTE-905/EV-303 was expected to be a positive trial because the study treatment is being compared with surgery alone and both systemic therapies are known to be highly active, but the hazard ratios (HRs) for event-free survival (EFS) and overall survival (OS) reported by researchers are particularly impressive in this vulnerable patient population,” says Dr Jonathan Rosenberg from the Memorial Sloan Kettering Cancer Center, New York, USA, discussing the study results.
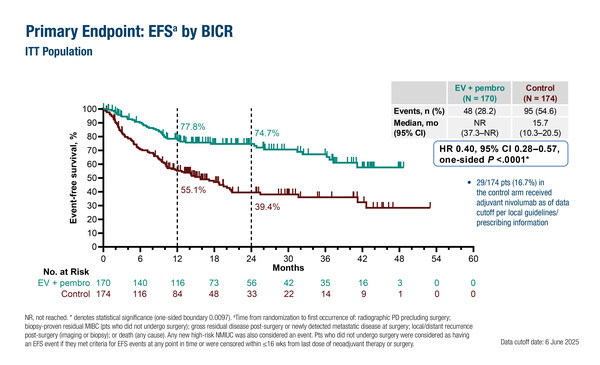
In the phase III KEYNOTE-905/EV-303 study, EV plus pembrolizumab significantly improved the primary endpoint, EFS (median not reached [NR] versus 15.7 months; hazard ratio [HR] 0.40; 95% confidence interval [CI] 0.28–0.57; p<0.0001), and OS (NR versus 41.7 months; HR 0.50; 95% CI 0.33–0.74; p=0.0002) when added to standard surgery compared with surgery alone in 344 cisplatin-ineligible patients with MIBC. The pathological complete response (pCR) rate was also significantly improved with peri-operative therapy (57.1% versus 8.6%; estimated difference 48.3%; 95% CI 39.5–56.5; p<0.000001), being much higher than that typically achieved with cisplatin-based neoadjuvant chemotherapy (Discov Oncol. 2025;16:1197).
“It is notable that only 67% of the patients who had a cystectomy and were randomised to EV plus pembrolizumab started adjuvant therapy,” notes Rosenberg. “It is important to understand why patients did not receive adjuvant treatment, and the relative importance of pre- and post-operative treatment. Circulating tumour DNA may provide some answers.”
While pre-operative cisplatin-based chemotherapy improves survival compared with radical surgery alone (BMC Urol. 2020;20:158), many patients (including those with impaired renal or cardiac function) are unable to tolerate this treatment and have few alternative options. Single-agent nivolumab is approved in the adjuvant setting based on the CheckMate-274 trial (N Engl J Med. 2021;384:2102–2114), but only for patients with high-risk, PD-L1-positive disease in the European Union. If the peri-operative EV plus pembrolizumab regimen becomes a new standard of care for cisplatin-ineligible MIBC, questions concerning first-line treatment in patients who progress to metastatic disease will need to be addressed. Rosenberg believes subsequent therapy may depend to some extent on the time to relapse: “If a patient relapses quickly, they should probably receive a different treatment, such as gemcitabine and carboplatin or cisplatin,” he suggests.
While peri-operative EV plus pembrolizumab did not affect the ability of study participants to undergo surgery, grade ≥3 treatment-emergent adverse events (AEs) occurred more frequently in the EV plus pembrolizumab arm compared with the control arm (71.3% versus 45.9%, respectively). Most frequently reported AEs of special interest were skin reactions peripheral neuropathy, ocular disorders and hyperglycaemia with EV, and hypothyroidism and severe skin reactions with pembrolizumab. “This treatment is not for everyone,” cautions Rosenberg. “For example, patients with severe peripheral neuropathy or poorly controlled diabetes are probably not good candidates for treatment with EV, while those with severe and active autoimmune conditions should probably not receive pembrolizumab.”
The treatment of MIBC continues to evolve rapidly. Additional insights on novel combination therapies will be provided by ongoing phase III trials in cisplatin-ineligible and -eligible MIBC, such as the VOLGA study that is currently exploring peri-operative durvalumab, tremelimumab and EV versus durvalumab plus EV compared with surgery alone in cisplatin-ineligible MIBC (NCT04960709), and the KEYNOTE-B15/EV-304 trial (NCT04700124) that is comparing pembrolizumab plus EV to cisplatin-based peri-operative chemotherapy in cisplatin-eligible patients.
Programme details
Vulsteke C, et al. Perioperative (periop) enfortumab vedotin (EV) plus pembrolizumab (pembro) in participants (pts) with muscle-invasive bladder cancer (MIBC) who are cisplatin-ineligible: The phase 3 KEYNOTE-905 study. ESMO Congress 2025 - LBA2