Longer duration of efficacy reported for encorafenib plus binimetinib as a treatment for NSCLC harbouring a BRAF V600 mutation
Findings from two studies presented at the ESMO Congress 2024 (Barcelona, 13–17 September) confirm the validity of targeting BRAF and MEK in BRAF V600E-mutant non-small cell lung cancer (NSCLC) for which two combinations of targeted agents – dabrafenib–trametinib and encorafenib–binimetinib – are now approved in Europe.
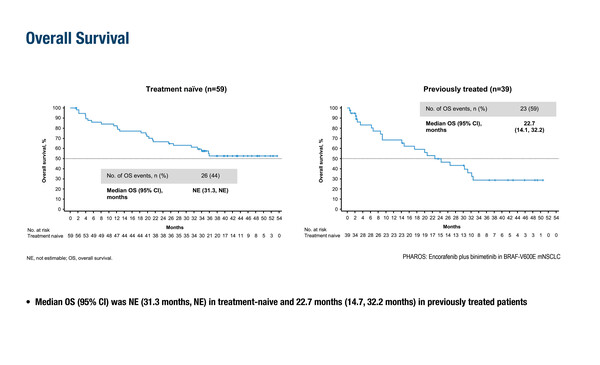
In the updated analysis from the single-arm, phase II PHAROS trial, which led to the approval of encorafenib combined with binimetinib (J Clin Oncol. 2023;41:3700–3711), following an additional 18 months of follow-up, the objective response rate (ORR) for treatment-naïve patients was 75% (95% confidence interval [CI] 62–85), with a median duration of response (DOR) of 40.0 months (95% CI 23.1–not estimable [NE]) (LBA56). For previously treated patients, the ORR was 46% (95% CI 30–63) and the median DOR was 16.7 weeks (95% CI 7.4–NR). Median progression-free survival (PFS) was 30.2 months (15.7–NE) after a median 33.3 months of follow-up in the treatment-naïve population, and 9.3 months (95% 6.2–32.3) after a median 14.0 months of follow-up in the previously treated population. Median overall survival (OS) was not estimable (95% CI 31.3–NE) in the treatment-naïve population and was 22.7 months (95% CI 14.1–32.2) in the previously treated population. Safety findings were consistent with the primary analysis, with treatment-related adverse events (TRAEs) leading to discontinuation in 26% of treatment-naïve patients and 16% of previously treated patients.
A second study, the ongoing, open-label, single-arm phase II IFCT-1904 ENCO-BRAF trial, is also evaluating encorafenib and binimetinib (Abstract 1259MO). Among 61 patients with previously untreated BRAF V600E-mutant NSCLC evaluable for efficacy, at a median follow-up of 18 months, the investigator-assessed ORR (primary endpoint) was 65.6% (95% CI 53.7–77.5), the median DOR was 13 months (95% CI 9.1–not reached [NR]) and the disease control rate was 85.2% (95% CI 76.3–94.1). The median PFS was 10.9 months (95% CI 6.4–16.7), while the median OS was not reached (95% CI 20.7–NR). The most frequent TRAEs were fatigue (42.2%), nausea (32.8%) and diarrhoea (31.3%) among 64 patients with available safety data. Permanent treatment discontinuation due to a TRAE occurred in 6 (9.4%) patients, while encorafenib and binimetinib dose reductions were reported for 34.4% and 32.8% of patients, respectively. Ocular events were also reported: blurred vision in 7 (10.9%) patients and serous/retinal detachment in 5 (7.8%) patients.
Commenting on these results, Dr Daniel Tan from the National Cancer Centre Singapore, says, “The findings from these two studies align with previous results using these targeted agents in NSCLC harbouring the BRAF V600E mutation. Overall, the rates of common side effects such as nausea and diarrhoea were similar to those observed with the dabrafenib–trametinib combination, although with encorafenib and binimetinib, there were lower incidences of dose reductions/interruptions and a notable absence of pyrexia events, providing a viable therapeutic option for BRAF-mutated NSCLC.”
Despite the encouraging results reported here, according to Tan a significant unmet need remains in the treatment of BRAF-mutant NSCLC. “One of the foremost challenges is to understand the mechanisms behind the resistance that inevitably develops to targeted therapies and identify precise strategies to overcome this challenge. The role of combinatorial approaches incorporating novel targeted therapies and immunotherapy warrant further investigation,” he concludes.
Programme details
Riely GJ, et al. Updated efficacy and safety from the phase II PHAROS study of encorafenib plus binimetinib in patients with BRAF V600E-mutant metastatic NSCLC (mNSCLC). ESMO Congress 2024, LBA56
Mini Oral Session – NSCLC metastatic, 14.09.2024, h. 10:15 – 11:45, Santander Auditorium – Hall 5
Planchard D, et al. Encorafenib plus binimetinib in patients (pts) with previously untreated BRAF V600E-mutant advanced non-small cell lung cancer (NSCLC): An open-label, multicenter phase II trial (IFCT-1904 ENCO-BRAF). ESMO Congress 2024, Abstract 1259MO
Mini Oral Session – NSCLC metastatic, 14.09.2024, h. 10:15 – 11:45, Santander Auditorium – Hall 5