The use of targeted agents in first and subsequent lines of therapy is improving outcomes in patients with mutated lung cancer progressing on standard chemotherapy, but a biomarker- or precision-guided approach is needed
Understanding and overcoming resistance mechanisms is a key strategy to maximising novel treatments for EGFR-mutant advanced non-small cell lung cancer (NSCLC), thus enabling use of agents beyond platinum chemotherapy and improving the use of first-line combinations. The results from several studies presented at the ESMO Congress 2024 (Barcelona, 13–17 September) further expand current knowledge of the mechanisms of resistance and treatment options in patients with EGFR-mutant NSCLC treated with such promising combinations, including osimertinib plus chemotherapy and amivantamab plus lazertinib.
In one presentation, the MARIPOSA investigators compared the mechanisms of treatment resistance among patients with EGFR-mutated advanced NSCLC receiving the EGFR–MET bispecific antibody amivantamab plus lazertinib, a third-generation tyrosine kinase inhibitor (TKI), compared with those from the osimertinib, using paired samples from 858 patients (LBA55). In the largest analysis of its kind, the researchers of this phase III study reported that patients receiving amivantamab and lazertinib were significantly less likely to develop MET amplification, secondary EGFR resistance mutations or small-cell transformation. Commenting on these results, Dr Natasha Leighl from the Princess Margaret Cancer Centre, Toronto, ON, Canada, says, “While the first two findings are due to the effective dual targeting of MET and EGFR by amivantamab and lazertinib, the reduced frequency of small-cell transformation is unexpected. This finding is clinically relevant and may help uncover some of the biology driving small cell transformation and identify future treatment options.” The study results contrast with evidence from the FLAURA2 study, where the mechanisms of resistance were similar between patients treated with osimertinib plus chemotherapy when compared with osimertinib alone (Ann Onc. 2023;34(Suppl_4):S1661–S1706). Leighl stresses the significance of the MARIPOSA study results: “These findings underscore the importance of molecular resistance testing at disease progression and will eventually have implications for subsequent treatment choices of chemotherapy, antibody–drug conjugates and other combinations.”
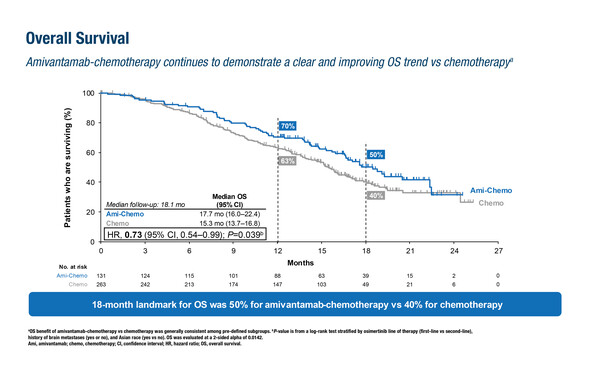
A previous report of a marked improvement in progression-free survival (PFS, hazard ratio [HR] 0.48) with a trend to overall survival (OS) benefit for amivantamab plus chemotherapy in the phase III MARIPOSA-2 study has led to a growing uptake of this regimen (Ann Oncol. 2024;35:77–90). As presented in Barcelona, second interim analysis and post-progression results of amivantamab plus chemotherapy (carboplatin–pemetrexed) versus chemotherapy alone in patients with EGFR-mutant advanced NSCLC that had progressed on osimertinib in the MARIPOSA-2 trial revealed a deepening survival benefit, though it did not reach the pre-specified significance threshold (LBA54). At a median follow-up of 18.1 months, OS in patients receiving amivantamab plus chemotherapy was 17.7 months compared with 15.3 months in those receiving chemotherapy alone (HR 0.73; p=0.039). PFS after the first subsequent therapy was significantly longer with amivantamab plus chemotherapy than with chemotherapy alone (median 16.0 months versus 11.6 months, respectively; HR 0.64; p=0.002). “These latest results continue to strengthen the position of amivantamab plus chemotherapy as the most effective regimen to date after disease progression on osimertinib, outperforming the previous standard of platinum chemotherapy alone,” says Leighl, although further confirmation is needed on its survival impact and its positioning following first-line combinations beyond osimertinib alone. “We eagerly await the final OS analysis, which will hopefully establish this regimen as providing a significant improvement in survival versus platinum doublet as second-line therapy. We also need to improve our management of toxicity, especially skin toxicity, optimise treatment delivery (e.g. with subcutaneous amivantamab) and clarify how this treatment option fits into a more personalised approach to osimertinib resistance based on molecular profiling.”
Adding further to the clinical picture to help determine optimised therapy in EGFR-mutated NSCLC, investigators from the REZILIENT1 phase IIb study presented the first data cut of the antitumour activity of zipalertinib, an irreversible and selective EGFR TKI, in patients with EGFR exon 20 insertion-mutated NSCLC that had progressed with amivantamab (Abstract 1254MO). In a group of 30 patients evaluable for efficacy, zipalertinib was associated with a 40% confirmed objective response rate with the median duration of response not yet estimable. “This degree of activity is similar to the initial report of a 41% response rate with zipalertinib in patients who had received prior platinum-based chemotherapy (J Clin Oncol. 2023;41:4218–55),” says Leighl. So far in the current analysis, the rate of dose reductions and discontinuations was low, and toxicity was manageable. “This report highlights the marked activity of zipalertinib even in patients with disease progression on amivantamab. Along with its favourable toxicity profile and previously reported central nervous system activity, zipalertinib is a very promising option for patients with EGFR exon20 insertion-mutated NSCLC even after amivantamab,” comments Leighl.
Leighl thinks that these latest results provide great hope for patients with EGFR-resistant NSCLC. “As the number of targeted agents and combinations increases, along with better patient outcomes, our future challenge will be to personalise first-line and subsequent treatment approaches. There are many considerations, including balancing efficacy and toxicity, front-line treatment combinations versus sequential approaches and how to maximise benefit using a biomarker or precision-guided approach. This new evidence helps us continue to improve our evidence-based approach to personalised, effective care for patients,” she concludes.
Programme details
Popat S, et al. Amivantamab plus chemotherapy vs chemotherapy in EGFR-mutated, advanced non-small cell lung cancer after disease progression on osimertinib: 2nd interim overall survival from MARIPOSA-2. ESMO Congress 2024, LBA54
Proffered Paper Session – NSCLC metastatic, 14.09.2024, h. 08:30 – 10:00, Barcelona Auditorium – Hall 2
Besse B, et al. Mechanisms of acquired resistance to first-line amivantamab plus lazertinib versus osimertinib in patients with EGFR-mutant advanced non-small cell lung cancer: An early analysis from the phase 3 MARIPOSA Study. ESMO Congress 2024, LBA55
Proffered Paper Session – NSCLC metastatic, 14.09.2024, h. 08:30 – 10:00, Barcelona Auditorium – Hall 2
Passaro A, et al. Safety and antitumor activity of zipalertinib in NSCLC patients (pts) with EGFR exon 20 insertion (ex20ins) mutations who received prior amivantamab. ESMO Congress 2024, Abstract 1254MO
Mini Oral Session – NSCLC metastatic, 14.09.2024, h. 10:15 – 11:45, Santander Auditorium – Hall 5