Final overall survival data from the CONTACT-02 study are negative, but a survival advantage was observed with the novel combination in patients with liver or bone metastasis
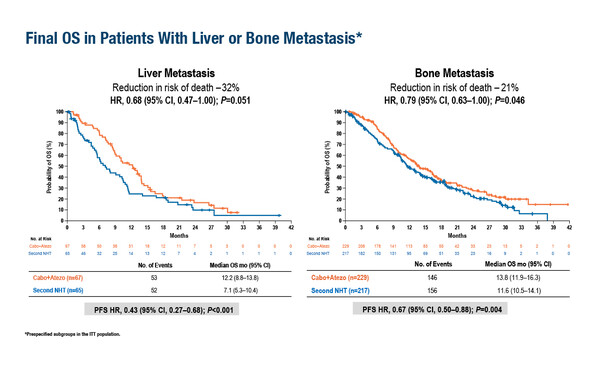
As reported at the ESMO Congress 2024 (Barcelona, 13–17 September), the final overall survival (OS) analysis of the phase III CONTACT-02 study in patients with metastatic castration-resistant prostate cancer (mCRPC) who progressed on a first novel hormonal therapy (NHT) and received cabozantinib plus atezolizumab or a second NHT did not achieve statistical significance (LBA67). No OS advantage from the experimental treatment was noted in the intention to treat population at a median follow-up of 24.0 months (14.8 months versus 15.0 months; hazard ratio [HR] 0.89; 95% confidence interval [CI] 0.72–1.10; p=0.30). However, a survival advantage was observed in some subgroups, including patients with liver (HR 0.68) or bone metastases (HR 0.79).
As previously reported, the study had met one of its primary endpoints, progression-free survival (PFS), which was significantly prolonged with cabozantinib plus atezolizumab compared with a second NHT (HR 0.65; 95% CI 0.50–0.84; p=0.0007) (J Clin Oncol. 2024;42).
Dr Elena Castro from Hospital Universitario 12 de Octubre, Madrid, Spain, believes the positive OS subgroup data from the CONTACT-02 study warrant further investigation in patients with liver metastases, although it will be challenging. “Patients with liver metastases tend to have aggressive disease and after progression to an NHT may be better served with chemotherapy than with a second NHT,” she says. Safety is also an important consideration for future trials. In the CONTACT-02 study, grade 3–4 treatment-related adverse events were reported in 40% of the cabozantinib plus atezolizumab arm (compared with 8% of the NHT arm).
Castro emphasises that, in the absence of significant clinical benefit, immune checkpoint inhibitors – either alone or in combination – may not be the best treatment choice for unselected populations of patients with mCRPC. However, for some patients, such as those with mismatch repair deficiency or high microsatellite instability, there is evidence of considerable clinical activity with immunotherapy (PLoS One. 2020;15:e0233260; Clin Genitourin Cancer. 2024;22:558–568; ESMO Congress 2024, LBA72).
“I do not think cabozantinib plus atezolizumab will become standard of care for all patients with mCRPC whose disease has progressed on NHT; however, it may be a good option for some patients,” she comments. “We need to improve on how to identify the right treatment at the right time for each patient,” she concludes.
Programme details
Agarwal N, et al. Cabozantinib (C) plus atezolizumab (A) versus 2nd novel hormonal therapy (NHT) in patients (Pts) with metastatic castration-resistant prostate cancer (mCRPC): Final overall survival (OS) results of the phase 3, randomized, CONTACT-02 study. ESMO Congress 2024, LBA67
Proffered Paper Session – GU tumours, prostate, 15.09.2024, h. 14:45 – 16:15, Valencia Auditorium – Hall 5
Mehra N, et al. Nivolumab 3mg/kg and ipilimumab 1mg/kg (nivo3/ipi1) in molecularly selected patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). ESMO Congress 2024, LBA72
Mini Oral Session – GU tumours, prostate, 16.09.2024, h. 10:15 – 11:45, Madrid Auditorium – Hall 2