Findings from two studies show high rates of complete remissions in patients with Bacillus Calmette-Guérin unresponsive high-risk non-muscle-invasive bladder cancer and muscle-invasive bladder cancer
At the ESMO Congress 2024 (Barcelona, 13–17 September), the novel investigational targeted releasing system, TAR 200, which is designed to provide prolonged intravesical delivery of gemcitabine into the bladder, was shown to be a potential treatment option for patients with Bacillus Calmette-Guérin (BCG)-unresponsive high-risk non-muscle-invasive bladder cancer (NMIBC) and those with muscle-invasive bladder cancer (MIBC)
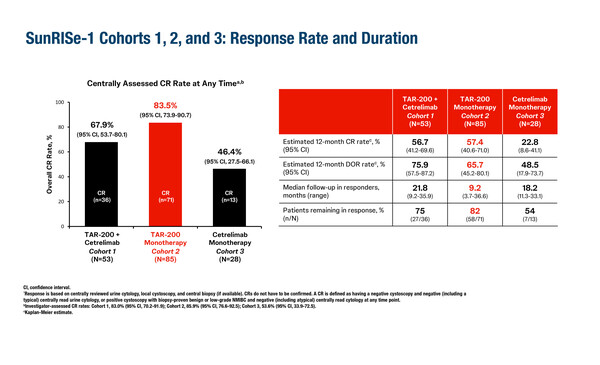
Updated results of the randomised, phase IIb SunRISe-1 study, showed that monotherapy with TAR-200 was more effective than TAR-200 combined with the anti-PD-1 agent cetrelimab or cetrelimab alone in patients with papillary BCG-unresponsive high-risk NMIBC (LBA85). The primary endpoint of centrally-confirmed complete response (CR) rate at any time through week 48 was 83.5% in the TAR-200 monotherapy cohort (n=71), 67.9% in the TAR-200 plus cetrelimab cohort (n=36) and 46.4% in the cetrelimab monotherapy cohort (n=13). Fewer patients reported at least one grade ≥3 treatment-related adverse event in the TAR-200 monotherapy cohort (9.4%) and in the cetrelimab monotherapy cohort (7.1%) than in the TAR-200 plus cetrelimab cohort (35.8%). These findings led the investigators to conclude that continued development of TAR-200 monotherapy rather than combination therapy in patients with BCG-unresponsive high-risk NMIBC is warranted. Of note, cetrelimab alone showed modest activity in this setting compared to other immune checkpoint inhibitors.
Also presented at the Congress, interim results from SunRISe-4 showed compelling efficacy of neoadjuvant TAR-200 plus cetrelimab in patients with MIBC (LBA84). The randomised phase II study compared TAR-200 plus cetrelimab with cetrelimab monotherapy in 84 patients with MIBC who were ineligible for or refused, neoadjuvant cisplatin-based chemotherapy. Pathological CR rates (defined as ypT0N0) in the subsequent cystectomy specimens were 42% in the TAR-200 plus cetrelimab cohort and 23% in the cetrelimab monotherapy cohort, while respective pathological overall response rates (defined as ≤ypT1N0) were 60% and 36%. The safety profile was reported as manageable; most adverse events were low grade.
“The past 5 years have seen immense positive changes in the treatments available for patients with BCG-unresponsive bladder cancer,” says Prof. Ashish Kamat from the University of Texas MD Anderson Cancer Center, Houston, TX, USA, “but we can clearly keep on raising the bar.” He adds, “The SunRIse studies are among several that are exploring alternative options to upfront radical cystectomy and the final results of SunRIse-1 could influence future clinical practice for patients with high-risk NMIBC.” There is a particular unmet need for treatments in patients with MIBC who are ineligible for or refuse neoadjuvant cisplatin-based chemotherapy. “For SunRIse-4 specifically, while these are interim results, the pathological CR rate of 42% with TAR-200 plus cetrelimab appears to be indicative of a true efficacy signal,” suggests Kamat. “The future goal would be to offer our patients bladder-sparing therapy. If clinical trials of other agents and/or exploration of an expanded indication of the combination of TAR-200 plus cetrelimab yield positive findings, then perhaps there is the potential that these regimens could be alternative options for patients,” he concludes.
Programme details
van der Heijden MS, et al. TAR-200 +/- cetrelimab (CET) and CET alone in patients (pts) with bacillus Calmette-Guérin-unresponsive (BCG UR) high-risk non–muscle-invasive bladder cancer (HR NMIBC): updated results from SunRISe-1 (SR-1). ESMO Congress 2024, LBA85
Mini Oral Session – GU tumours, non-prostate, 15.09.2024, h. 08:30 – 10:00, Bilbao Auditorium – Hall 2
Necchi A, et al. TAR-200 plus cetrelimab (CET) or CET alone as neoadjuvant therapy in patients (pts) with muscle-invasive bladder cancer (MIBC) who are ineligible for or refuse neoadjuvant cisplatin-based chemotherapy (NAC): interim analysis of SunRISe-4 (SR-4). ESMO Congress 2024, LBA84
Proffered Paper Session 2 – GU tumours, non-prostate, 16.09.2024, h. 08:30 – 10:00, Sevilla Auditorium – Hall 2