Practice changing results of event-free survival and overall survival are reported from the NIAGARA study
At the ESMO Congress 2024 (Barcelona, 13–17 September), results presented at a Presidential Symposium show for the first time the benefits of an immunotherapy-based treatment, with durvalumab extending survival compared with neoadjuvant chemotherapy alone in patients with muscle-invasive bladder cancer (MIBC) (LBA5)
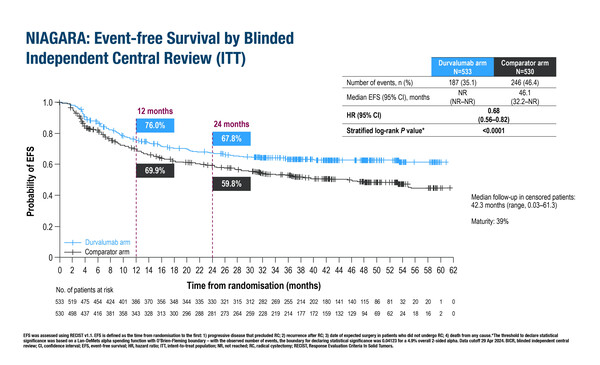
In a pre-planned interim analysis of the phase III NIAGARA trial, adding durvalumab to neoadjuvant chemotherapy (NAC) before radical cystectomy and durvalumab monotherapy after surgery produced significant and clinically meaningful improvements in event-free survival (EFS) and overall survival (OS) compared with NAC alone. Importantly, the addition of neoadjuvant durvalumab did not compromise the ability to complete radical cystectomy.
The trial recruited 1,063 patients eligible for cisplatin treatment and planned to undergo radical cystectomy. Patients were randomised (1:1) to neoadjuvant durvalumab plus NAC (cisplatin plus gemcitabine) followed by radical cystectomy then adjuvant durvalumab monotherapy, or NAC followed by radical cystectomy alone. Compared with NAC plus surgery, EFS (primary endpoint) in the durvalumab arm was significantly longer (hazard ratio [HR] 0.68; 95% confidence interval [CI] 0.56–0.82; p<0.0001), as was OS (HR 0.75; 95% CI 0.59–0.93; p=0.0106). Radical cystectomy was completed in 88% of patients in the durvalumab arm and in 83% in the NAC plus surgery arm.
Commenting on this clinical trial and its findings, Prof. Petros Grivas from the University of Washington and Fred Hutchinson Cancer Center, Seattle, WA, USA, says, “The NIAGARA trial is practice changing for patients with localised MIBC as the trial met its primary endpoint of EFS, while OS was also significantly extended, which is a more difficult endpoint to meet (of note, OS can be confounded by access to and effectiveness of subsequent therapies).” Grivas is reassured by the safety data. “The toxicity profile was manageable and as expected for checkpoint inhibitors and chemotherapy, with no synergistic toxicity or new safety concerns,” he says, and thinks that we should evaluate in more detail adverse events, quality of life, patient reported outcomes and healthcare utilisation during the adjuvant durvalumab therapy period; moreover Grivas says that patients should be carefully educated about the risk of immune-related adverse events and be asked to report them in a timely and accurate manner.
Grivas notes that there are still a number of unanswered questions on the overall position of immunotherapy for patients with localised MIBC. “Currently, there is an active debate about whether immunotherapy should be used in the neoadjuvant and/or adjuvant treatment setting. Furthermore, we need to understand whether patients with pathologic complete response need adjuvant immunotherapy or not, and what the clinical utility of circulating tumour DNA is in this setting. Updated data from CheckMate 274 and AMBASSADOR phase III adjuvant trials, as well as the results of ongoing peri-operative phase III trials in this population, e.g. NCT03661320, KEYNOTE-866, KEYNOTE-905/EV-303, KEYNOTE-B15/EV-304 and VOLGA, will assist in this ongoing discussion and provide very important data in the future,” he concludes.
Programme details
Powles TB, et al. A randomized phase 3 trial of neoadjuvant durvalumab plus chemotherapy followed by radical cystectomy and adjuvant durvalumab in muscle-invasive bladder cancer (NIAGARA). ESMO Congress 2024, LBA5
Presidential Symposium II – Practice-changing trials, 15.09.2024, h. 16:30 – 17:50, Barcelona Auditorium – Hall 2