Three-year disease-free survival data from NICHE-2 and findings from NICHE-3 add to current knowledge on the benefits of immune checkpoint inhibition in this setting
Three-year disease-free survival (DFS) results from the NICHE-2 study presented at the ESMO Congress 2024 (Barcelona, 13–17 September) provide further evidence for the benefit of neoadjuvant immunotherapy in the treatment of mismatch repair-deficient (dMMR) colon cancer (LBA24).
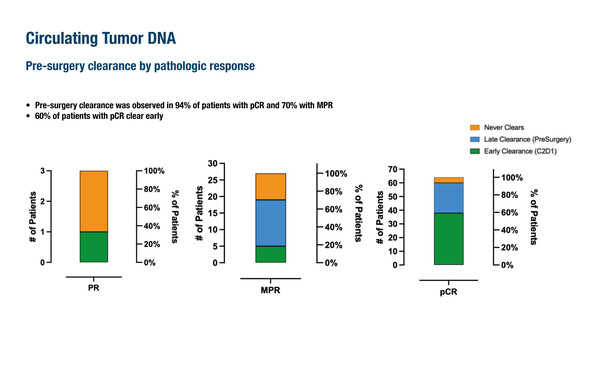
In the study, three-year DFS – one of the two co-primary endpoints – was 100% in 111 patients with dMMR, locally advanced colon cancer who received a single dose of ipilimumab and two doses of nivolumab followed by surgery within 6 weeks, surpassing the 93% DFS rate deemed successful. On day 15, 45% of 102 patients with available plasma samples had circulating tumour DNA (ctDNA) clearance – down from 92% at baseline. Prior to surgery, 16 patients remained ctDNA-positive, including 5 with pathological complete response (pCR), 8 with a major pathological response (MPR), 2 with progressive disease and 1 non-responder. In addition, ctDNA clearance was reported in 94% of patients with a pCR and in 70% of those with an MPR. At the minimal residual disease analysis time point of 3 weeks post-surgery, all patients were negative for ctDNA.
Previously, the NICHE-2 study was shown to have met its safety endpoint with 4% of patients having grade 3–4 immune-related adverse events and 2 patients experiencing a delay in surgery of 2 weeks or more. In the initial report from the study at a median follow-up of 13.1 months, there were no cases of disease recurrence and again in the current presentation at a median 36.5 (range 7.8–83.4) months after surgery, there were no reports of disease recurrence.
Additionally, results from the NICHE-3 study of two doses each of nivolumab and relatlimab given prior to surgery within 8 weeks of enrolment in patients with locally advanced dMMR colon cancer revealed a pathological response (≤50% residual viable tumour) rate of 97% in 59 patients who had undergone surgery (Abstract 503O). This included an MPR rate of 92% and pCR rate of 68%. Grade 3–4 immune-related adverse events were reported in 6 (10%) patients, causing surgery to be delayed in 3 patients. Long-term endocrine disorders were reported: thyroid dysfunction (22%), including one grade 3 event (2%), and adrenal insufficiency (8%).
Programme details
Chalabi M, et al. Neoadjuvant immunotherapy in locally advanced MMR-deficient colon cancer: 3-year disease-free survival from NICHE-2. ESMO Congress 2024, LBA24
Proffered Paper Session 1 – GI tumours, lower, 15.09.2024, h. 08:30 – 10:00, Sevilla Auditorium – Hall 2
de Gooyer PGM, et al. Neoadjuvant nivolumab (nivo) plus relatlimab (rela) in MMR-deficient colon cancer: Results of the NICHE-3 study. ESMO Congress 2024, Abstract 503O
Proffered Paper Session 1 – GI tumours, lower, 15.09.2024, h. 08:30 – 10:00, Sevilla Auditorium – Hall 2