Preliminary results from three clinical trials reveal some benefits of cetuximab plus irinotecan, amivantamab plus FOLFOX or FOLFIRI and ramucirumab plus standard of care for patients with mCRC, including after progression on standard therapies
Despite the advent of individualised treatment based on prognostic and predictive molecular markers, metastatic colorectal cancer (mCRC) remains a disease with poor prognosis, with a 5-year survival rate of 14% (World J Gastroenterol. 2023;29:1569–1588) and there is an urgent need for novel treatment options for patients who progress on standard therapies. At the ESMO Congress 2024 (Barcelona, 13–17 September), results were presented from three clinical trials that evaluated novel treatment strategies for this disease, providing some promising findings.
In the CITRIC phase II study, patients with mCRC who had previously benefited from first-line anti-EGFR therapy but had progressed on second-line treatment and were triple-negative for RAS, BRAF and EGFR ectodomain mutations were randomised to receive the EGFR inhibitor cetuximab combined with irinotecan (n=31) or investigator’s choice of therapy (control arm; n=27) (Abstract 511MO). Compared with the control arm, patients treated with cetuximab plus irinotecan showed a trend towards a higher objective response rate (ORR) (12.9% versus 0%; p=0.116) and significantly improved disease control rate (DCR; 74.2% versus 44.4%; p=0.031). Median progression-free survival (PFS) was 4.41 months for the cetuximab plus irinotecan arm compared with 2.24 months for the control arm (hazard ratio [HR] 0.717; 95% confidence interval [CI] 0.403–1.276; p=0.255). Commenting on these findings, Dr Sara Lonardi from the Veneto Institute of Oncology, Italy, says, “Although the ORR is not particularly impressive, the data showing superior DCR and a trend for prolonged PFS obtained from a randomised trial contribute to a growing body of evidence supporting rechallenge with EGFR inhibitors in this molecularly selected patient population. Clinically, this strategy represents a valuable opportunity for patients.”
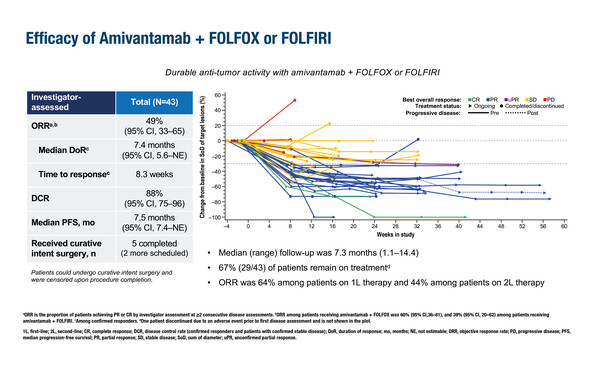
In a second study, the safety and efficacy of the EGFR–MET bispecific antibody amivantamab combined with either 5-fluorouracil–folinic acid–oxaliplatin (FOLFOX) or 5-fluorouracil–folinic acid–irinotecan (FOLFIRI) was evaluated. The OrigAMI-1 phase Ib/II study involved 43 patients with RAS/RAF wild-type mCRC who were EGFR inhibitor-naïve (Abstract 513MO). After a median follow-up of 7.3 months, the ORR was 49% (95% CI 33–65), DCR was 88% (95% CI 75–96) and median PFS was 7.5 months (95% CI 7.4–not estimable). Among patients with measurable liver lesions (n=30), the intrahepatic ORR and DCR were 53% and 93%, respectively. Seven (16%) patients proceeded to surgery with curative intent. “These data are promising and provide a strong foundation for further research,” notes Lonardi. “It is also encouraging that there were no unexpected or unmanageable side effects with these drug combinations. Additionally, the reduced treatment administration time of the subcutaneous formulation that will be explored in OrigAMI-2 and -3 offers the potential to improve patient quality of life and reduce the burden on healthcare resources.”
Finally, the RAMTAS/IKF643 phase III trial investigated the benefits of adding the anti-angiogenic agent ramucirumab to the current standard of care (trifluridine plus tipiracil) in 428 patients with chemotherapy-refractory mCRC (LBA25). The median overall survival (OS), the primary endpoint, was 7.46 months for patients treated with ramucirumab compared with 7.06 months for patients on standard of care only (HR 0.871; 95% CI 0.708–1.073; p=0.1941). Improvements in OS were observed in female patients (HR 0.71; 95% CI 0.52–0.98; p=0.04) and those with left-sided tumours (HR 0.77; 95% CI 0.60–1.070; p=0.05). Median PFS was significantly prolonged with ramucirumab compared with standard of care alone (2.37 months versus 2.07 months; HR 0.774; 95% CI 0.636–0.949; p=0.011) and there was also a significant improvement in DCR (39.4% versus 31.6%; p=0.0336). “Although this study did not meet its primary endpoint, this is not surprising given the challenge of detecting improvements in OS in such a heavily pre-treated patient population,” remarks Lonardi. “The subgroup findings are scientifically interesting but are unlikely to impact clinical decisions without further study.”
“The future of mCRC treatment lies in molecularly targeted therapies and several promising agents are currently in development. However, maintaining quality of life and functional status is equally crucial to ensure that patients remain eligible for new therapies as they become available,” she concludes.
Programme details
Santos Vivas C, et al. Third line rechallenge with cetuximab (Cet) and irinotecan in circulating tumor DNA (ctDNA) selected metastatic colorectal cancer (mCRC) patients: The randomized phase II CITRIC trial. ESMO Congress 2024, Abstract 511MO
Mini Oral Session – GI tumours, lower, 14.09.2024, h. 14:45 – 16:15, Granada Auditorium – Hall 6
Pietrantonio F, et al. Amivantamab plus FOLFOX or FOLFIRI in metastatic colorectal cancer: Results from OrigAMI-1, an open-label, phase Ib/II study. ESMO Congress 2024, Abstract 513MO
Mini Oral Session – GI tumours, lower, 14.09.2024, h. 14:45 – 16:15, Granada Auditorium – Hall 6
Kasper-Virchow S, et al. Randomized phase III trial of ramucirumab in combination with TAS102 (Trifluridin/Tipiracil) vs. TAS102 monotherapy in heavily pretreated metastatic colorectal cancer: The RAMTAS/IKF643 trial of the German AIO (AIO-KRK-0316). ESMO Congress 2024, LBA25
Proffered Paper Session 2 – GI tumours, lower, 15.09.2024, h. 14:45 – 16:15, Madrid Auditorium – Hall 2