Final late-phase trial results show that the addition of pre-operative chemoradiotherapy to peri-operative chemotherapy does not improve long-term outcomes in patients
Results presented at the ESMO Congress 2024 (Barcelona, 13–17 September) demonstrate that adding pre-operative chemoradiotherapy (CRT) to peri-operative chemotherapy improved pathological outcomes but not overall survival (OS) in a randomised, phase III trial in patients with resectable gastric and gastro-oesophageal cancer (LBA58).
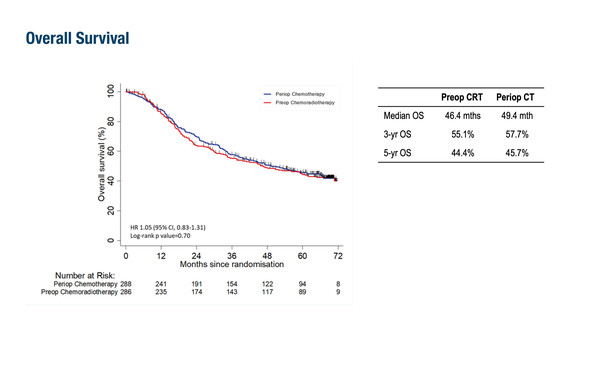
Peri-operative chemotherapy with fluorouracil–leucovorin–oxaliplatin–docetaxel is one current standard of care for patients with resectable gastric cancer. However, this regimen is not curative for a large proportion of patients, emphasising the need to develop novel treatment strategies (Int J Mol Sci. 2023;24:4877). The TOPGEAR study investigated the impact of adding pre-operative CRT to peri-operative chemotherapy in 574 patients diagnosed with resectable gastric and gastro-oesophageal adenocarcinomas. Compared with patients who received peri-operative chemotherapy alone, patients who received pre-operative CRT had a higher pathological complete response rate (pCR; 16.8% versus 8.0%, respectively; p<0.0001), a higher rate of major pathological response (<10% residual tumour: 32.7% versus 21.3%, respectively), and greater tumour downstaging following resection. However, after a median follow-up of 66.7 months, there was no significant difference in OS or progression-free survival (PFS) for the peri-operative chemotherapy versus pre-operative CRT arms (OS: 49.4 months versus 46.4 months, respectively; PFS: 31.8 months versus 31.4 months, respectively).
According to Dr Elizabeth Smyth from the Oxford University Hospitals NHS Foundation Trust, UK, the findings from this large, global, investigator-led study align with those from the ESOPEC study (J Clin Oncol. 2024;42:LBA 1). “They confirm that there is no role for pre-operative CRT in the treatment of operable gastro-oesophageal adenocarcinoma. While CRT appeared to be effective in targeting the local tumour, it did not address distant metastases, which remain the primary cause of mortality,” she says.
Smyth highlights that with a rapidly evolving landscape of targeted and immunotherapy drugs available for patients with advanced gastro-oesophageal adenocarcinoma, there is an urgent need to integrate these helpful approaches into treatment strategies for patients with operable upper gastrointestinal malignancies.
One of the challenges with investigating new treatments for operable gastric cancer is the lengthy duration of peri-operative trials. We need to complete these trials more quickly so that we can accelerate the delivery of beneficial therapies to patients,” she concludes. “A key strategy will be to develop new surrogate endpoints, such as digital pathology or circulating tumour DNA, that are more predictive of long-term outcomes compared with the current tumour response grading system.”
Programme details
Leong T, et al. A randomized phase III trial of perioperative chemotherapy (periop CT) with or without preoperative chemoradiotherapy (preop CRT) for resectable gastric cancer (AGITG TOPGEAR): final results from an intergroup trial of AGITG, TROG, EORTC and CCTG. ESMO Congress 2024, LBA58
Proffered Paper Session 2 – GI tumours, upper digestive, 14.09.2024, h. 08:30 – 10:00, Madrid Auditorium – Hall 2