Final analysis of the KEYNOTE-811 trial reports positive findings when adding pembrolizumab to trastuzumab and chemotherapy in treatment-naïve patients
As presented at the ESMO Congress 2024 (Barcelona, 13–17 September), the final per protocol analysis of the KEYNOTE-811 trial shows an overall survival (OS) benefit with first-line pembrolizumab versus placebo added to trastuzumab and chemotherapy in patients with treatment-naïve unresectable, HER2-positive metastatic gastric/gastro-oesophageal junction (G/GEJ) adenocarcinoma (Abstract 1400O).
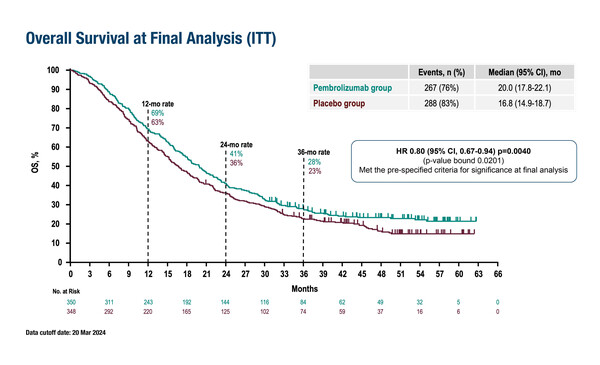
In a total of 698 patients, first-line pembrolizumab added to trastuzumab and chemotherapy was associated with a median OS of 20.0 months versus 16.8 months for placebo added to trastuzumab and chemotherapy (hazard ratio [HR] 0.80; 95% confidence interval [CI] 0.67–0.94; p=0.004) at a median follow-up of 50.2 months. In an analysis of 594 patients with a PD-L1 combined positive score ≥1, median OS was 20.1 months in the pembrolizumab group versus 15.7 months in the control group (HR 0.79; 95% CI 0.66–0.95).
Median progression-free survival continued to be longer with the pembrolizumab combination than with the comparator in the overall population (10.0 months versus 8.1 months; HR 0.73; 95% CI 0.61–0.87) and in those with a PD-L1 combined positive score ≥1 (10.9 months versus 7.3 months; HR 0.72; 95% CI 0.60–0.87).
These data have been anticipated by positive findings from interim analyses of KEYNOTE-811 presented at the ESMO Congress 2023, and published subsequently (Lancet. 2023;402:2197−2208). “However, this is the first time that formal testing has been performed on the co-primary endpoint of OS and the fact that patients gained around 3 months is clinically meaningful,” comments Dr Filippo Pietrantonio from the Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy. “The OS benefit – 4.4 months gained – was even greater in the large subgroup with a PD-L1 combined positive score ≥1 and this fits with current approved indications,” he notes.
In upper gastrointestinal cancers, the HER-positive treatment landscape is evolving quickly with several combinations currently under investigation. “From now on, when any of these novel combinations are ready to move into first-line phase III testing, pembrolizumab–trastuzumab–chemotherapy will be the standard-of-care comparator arm,” Pietrantonio concludes. “As a next step, it will be important to test the KEYNOTE-811 combination in earlier stage G/GEJ adenocarcinoma to help move on from chemotherapy as the current standard of care.”
Programme details
Janjigian YY, et al. Final overall survival for the phase 3, KEYNOTE-811 study of pembrolizumab plus trastuzumab and chemotherapy for HER2+ advanced, unresectable or metastatic G/GEJ adenocarcinoma. ESMO Congress 2024, Abstract 1400O
Proffered Paper Session 2 – GI tumours upper digestive, 14.09.2024, h. 08:30 – 10:00, Madrid Auditorium – Hall 2