Adding immunotherapy to standard of care shows benefit compared with placebo and is confirmed to be practice-changing for this patient population
Pembrolizumab plus standard of care (SOC; trastuzumab plus chemotherapy) has been recently approved in Europe for the treatment of HER2-positive metastatic gastric or gastro-oesophageal junction (G/GEJ) adenocarcinoma, based on responses in the phase III KEYNOTE-811 trial (Nature. 2021;600:727–730).
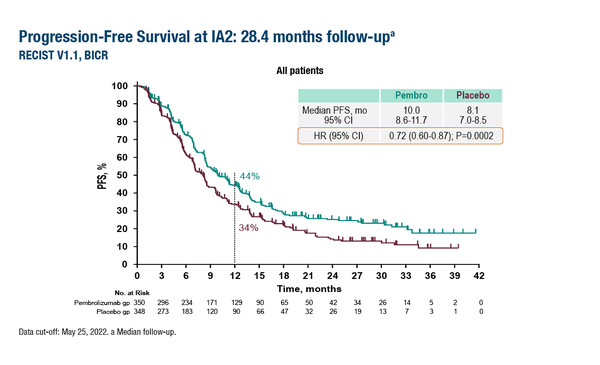
In the interim progression-free survival (PFS) analysis of the trial, presented at the ESMO Congress 2023 (Madrid, 20–24 October), first-line pembrolizumab plus SOC significantly improved PFS versus placebo plus SOC in all patients (median PFS: 10.0 months versus 8.1 months; hazard ratio [HR] 0.72; 95% confidence interval [CI] 0.60–0.87; p=0.0002) and in patients with a PD-L1 combined positive score ≥1 (10.8 months versus 7.2 months; HR 0.70; 95% CI 0.58–0.85) at a median follow-up of 28.4 months (Abstract 1511O). In the third interim analysis after 606 PFS events and at a median follow-up of 38.5 months, 24-month PFS rates were 24% and 15%, respectively.
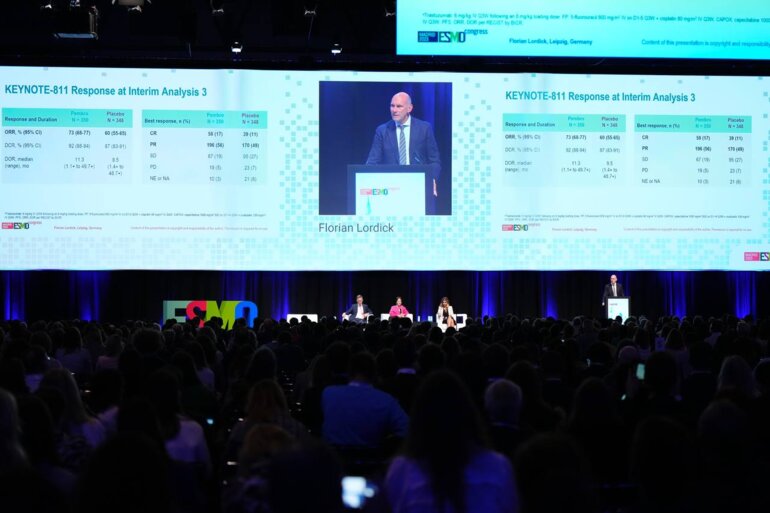
In the third interim analysis, the objective response rate was also improved with pembrolizumab plus SOC compared with placebo plus SOC (73% versus 60%), with respective disease control rates of 92% versus 87% lasting a median duration of 11.3 months versus 9.5 months.
Commenting on the study and its findings, Prof. Florian Lordick from the University of Leipzig, and University Cancer Center Leipzig, Germany notes that these data have been long awaited and will change the treatment landscape for patients with HER2-positive advanced G/GEJ cancer.
“The longer PFS and durable tumour responses observed with pembrolizumab plus SOC compared with placebo plus SOC are sufficient to prove the benefits of the combination therapy in a patient population where symptom burden is high and any response to treatment may result in a considerable improvement in quality of life,” he explains. “From a regulatory perspective, PFS should be considered a valuable endpoint in this setting, with no need to wait for overall survival data.”
Lordick comments that, “It will be interesting to see additional details of the sub-analysis based on PD-L1, as there might be a small sub-group of patients with HER2-positive, PD-L1-negative G/GEJ cancer in whom the pembrolizumab combination will not become the new standard. However, previous research indicates that PD-L1 is co-expressed in the majority of patients with HER2-positive G/GEJ tumours.”
Abstract discussed:
Janjigian YY, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Survival results from the phase 3, randomized, double-blind, placebo-controlled KEYNOTE-811 study. ESMO Congress 2023, Abstract 1511O
Proffered Paper Session 1 – Gastrointestinal tumours, upper digestive, 20.10.2023, h. 14.00 – 15.30, Barcelona Auditorium – Hall 9