Positive data presented from the LEAP-012 study for intermediate-stage disease, but it is still unclear how these will influence clinical practice
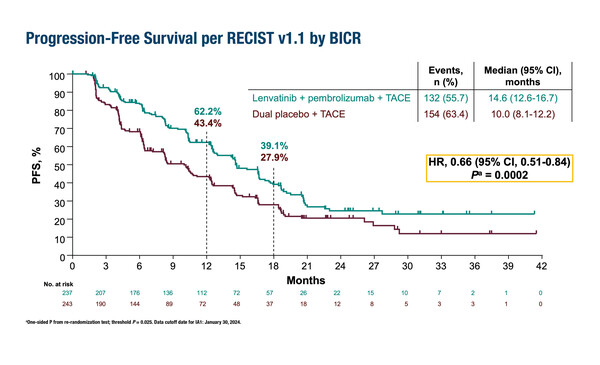
A combination of transarterial chemoembolization (TACE), lenvatinib and pembrolizumab significantly improved progression-free survival (PFS), one of the dual primary endpoints, compared with TACE alone in patients with intermediate-stage hepatocellular carcinoma (HCC), according to a first interim analysis of the phase III LEAP-012 study presented at the ESMO Congress 2024 (Barcelona, 13–17 September). With a median time from randomisation to data cut-off of 25.6 months, median PFS was 14.6 months versus 10.0 months, respectively (hazard ratio [HR] 0.66; 95% confidence interval [CI] 0.51–0.84; p=0.0002 [significance threshold p=0.025]) (LBA3).
TACE is the standard-of-care therapy for intermediate-stage HCC, but while it can prolong the disease course, it is not a curative treatment and most patients eventually progress. “There is an important unmet need to improve the results of locoregional treatment in this setting, and several ongoing trials are exploring combinations with systemic therapy,” comments Prof. Lorenza Rimassa from the Humanitas University and IRCCS Humanitas Research Hospital, Milan, Italy.
Overall survival (OS) data were immature, and the significance threshold (p=0.025) was not met (HR 0.80; 95% CI 0.57–1.11; p=0.0867). “As survival times tend to be relatively long with this disease stage, it is acceptable to have an interim analysis of PFS as this gives us an insight into the results without waiting for the survival data,” notes Rimassa. “As patients in the TACE arm will receive systemic therapies upon progression, the OS data may not be positive even with a longer follow-up, but the study showed a statistically significant and clinically meaningful improvement in PFS and an early trend toward improvement in OS in the combination arm.”
The lenvatinib plus pembrolizumab combination was previously evaluated in advanced HCC (LEAP-002 trial; Lancet Oncol. 2023;24:1399–1410), but did not meet pre-specified significance for improved OS and PFS compared with lenvatinib plus placebo. According to Rimassa there are a number of possible reasons for this, including the higher-than-expected activity in the lenvatinib arm (OS was 19 months). She does not expect the results of LEAP-002 to negatively impact the future use of the combination in earlier stages of HCC, given that the systemic therapy combined with TACE has demonstrated activity in intermediate-stage HCC in the LEAP-012 study, and encouraging results are also being reported with other immunotherapy combinations in early-stage disease. “The stages of HCC are very different, so it is difficult to predict the activity of a regimen from one stage to another, but I think treatment of earlier-stage disease will differ from that for advanced HCC,” she says.
Rimassa believes that, “The LEAP-012 study could influence future clinical practice given the positive PFS data, although longer follow-up will be needed and OS data, if possible.”
The safety profile of the lenvatinib plus pembrolizumab combination will also be key to its future use as patients with intermediate-stage HCC do not typically experience adverse effects between TACE procedures. In the LEAP-012 study, grade 3–5 treatment-related adverse events (TRAEs) occurred in 73.0% of patients in the lenvatinib plus pembrolizumab arm compared with 31.5% of the TACE-only group, and TRAEs led to the discontinuation of both study drugs in 8.4% versus 1.2% of patients, respectively. “I think that there will be patients with intermediate-stage HCC who will be candidates for this combination therapy – it will give them another option – but not all patients will receive systemic therapy plus TACE. Our task will be to identify which patients could benefit the most from this combination therapy,” she concludes.
Programme details
Llovet J, et al. Transarterial chemoembolization (TACE) with or without lenvatinib (len) + pembrolizumab (pembro) for intermediate-stage hepatocellular carcinoma (HCC): phase 3 LEAP-012 study. ESMO Congress 2024, LBA3
Presidential Symposium I – Practice-changing trials, 14.09.2024, h. 16:30 – 18:15, Barcelona Auditorium – Hall 2