However, the future of TKI combinations for these rare tumours is uncertain
In advanced gastrointestinal stromal tumours (GIST), combination tyrosine kinase inhibitor (TKI) therapy is the established standard of care, but resistance to approved agents can develop quickly. At the ESMO Congress 2024 (Barcelona, 13–17 September), findings from three trials evaluating the efficacy and safety of novel TKIs and treatment combinations for advanced GIST report some promising data, but also raise some questions on the future of combining TKIs for this population.
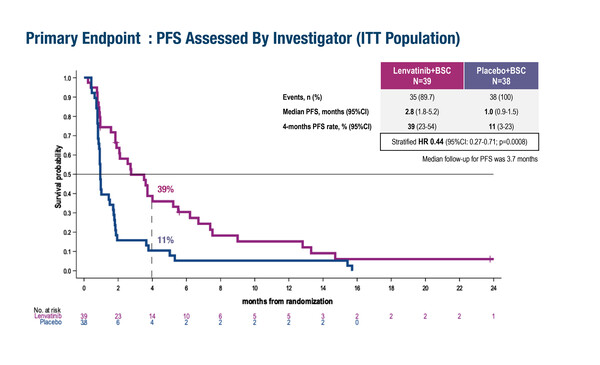
Results from the LENVAGIST phase II study showed that after 4 months of treatment, the progression-free survival (PFS) rate was 39% (95% confidence interval [CI] 23–54) in patients receiving the broad spectrum TKI lenvatinib compared with 11% (95% CI 3–23) in those treated with placebo (hazard ratio [HR] 0.44; 95% CI 0.27–0.71; p=0.0008) (LBA79). The study enrolled patients with GIST that had progressed on imatinib and sunitinib, with patients randomised to lenvatinib (n=39) or placebo (n=38). After a median follow-up of 22.1 months, median overall survival (OS) was 14.4 months (95% CI 7.1–18.9) for the lenvatinib group versus 8.7 months (95% CI 5.2–14.4) for placebo (HR 0.77; 95% CI 0.46–1.28; p=0.31). Grade ≥3 treatment-related adverse events (AEs) were reported in 56.4% of patients in the lenvatinib arm and 18.4% in the placebo arm. Commenting on the results, Prof. Sebastian Bauer from University Hospital Essen, Germany, says, “In this trial, the benefits of lenvatinib appear to be broadly consistent with those shown previously for regorafenib in the third-line setting in the GRID trial (Lancet. 2013;381:295–302). It will be interesting to learn more about the grades 1 and 2 AEs in this study as these are particularly important to patients, and also to gain an understanding of how active this treatment is against specific secondary mutations.”
The single-arm phase II AXAGIST study of the VEGF inhibitor axitinib combined with the checkpoint inhibitor avelumab for patients with advanced GIST showed that in 56 patients with heavily pre-treated disease, 5 patients (8.9%) had a partial response, 34 patients (60.7%) had stable disease, and 17 patients (30.4%) had disease progression (Abstract 1723MO). More than half (53.4%) of patients had received three prior lines of TKI therapy. The median PFS and OS were 4.6 months (95% CI 2.9–6.4) and 14.2 months (95% CI 9.2–26.3), respectively. Grade ≥3 treatment-related AEs were reported by 30.4% of patients, with 3.6% of patients discontinuing both avelumab and axitinib owing to treatment-related AEs. "The median PFS is shorter than that achieved in the INVICTUS trial, which investigated ripretinib in the fourth-line setting (Lancet Oncol. 2020;21:923–934), indicating that the addition of a checkpoint inhibitor appears not to be particularly beneficial in these patients,” notes Bauer. “We now need to find out more about how the clinical results correlate with biomarker data from these patients and whether this combination is more efficacious for particular subsets of patients with GIST.”
Findings for patients with succinate dehydrogenase (SDH)-deficient GIST, a rare disease with limited treatment options, who received the TKI olverembatinib in a phase I study were also presented, showing that treatment was generally well-tolerated and led to a partial response in 6 patients (23.1%) (Abstract 1722MO). Patients in this study were heavily pre-treated, with 50% having received ≥3 TKIs. The median PFS was 22.0 months (95% CI 12.9–38.6). According to Bauer it was a great achievement to complete a trial in this rare subgroup and the results with this agent are more promising than have been seen in earlier studies in these patients.
He concludes, “Although the results presented here are interesting, particularly for the patients with SDH-deficient GIST, the data for lenvatinib and for the axitinib and avelumab combination are unlikely to be sufficiently compelling to support a registrational clinical trial. I anticipate that the next generation of clinical trials for GIST will focus on more specific KIT-targeted inhibitors and the use of rational combinations of treatments.”
Programme details
Qiu H, et al. Updated efficacy results of olverembatinib (HQP1351) in patients with succinate dehydrogenase (SDH)-deficient gastrointestinal stromal tumors (GIST) and potential mechanisms of action (MOA). ESMO Congress 2024, Abstract 1722MO
Mini Oral Session – Sarcoma, 13.09.2024, h. 16:00 – 17:30, Alicante Auditorium – Hall 3
Rutkowski P, et al. Axitinib plus avelumab in patients with unresectable/metastatic gastrointestinal stromal tumor (GIST) after failure of standard therapy: Single-arm phase II study (AXAGIST). ESMO Congress 2024, Abstract 1723MO
Mini Oral Session – Sarcoma, 13.09.2024, h. 16:00 – 17:30, Alicante Auditorium – Hall 3
Le Cesne A, et al. LENVAGIST: A multicentre, comparative, placebo (P)-controlled, double-blinded, phase II study of the efficacy of lenvatinib (L) in patients with advanced GIST after failure of imatinib and sunitinib. ESMO Congress 2024, LBA79
Proffered Paper Session – Sarcoma, 16.09.2024, h. 14:45 – 16:15, Santander Auditorium – Hall 5