Phase III trial findings reveal significant improvements in PFS and OS rates, although the population studied may not be wholly indicative of high-risk disease
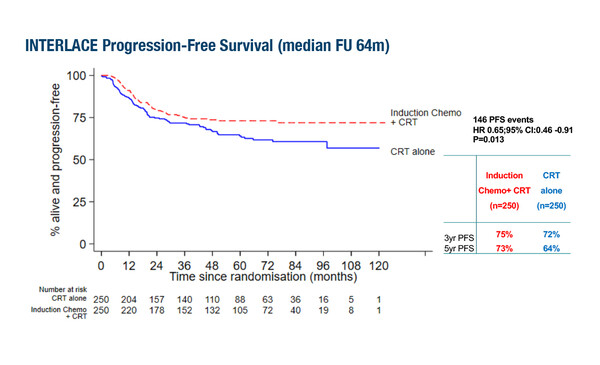
Results from the GCIG INTERLACE trial presented in a Presidential Symposium at the ESMO Congress 2023 (Madrid, 20–24 October) showed a progression-free survival (PFS) rate of 73% and an overall survival (OS) rate of 80% at 5 years with induction chemotherapy prior to chemoradiotherapy (CRT) compared to rates of 64% (hazard ratio [HR] 0.65; 95% confidence interval [CI] 0.46–0.91; p=0.013) and 72% (HR 0.61; 95% CI 0.40–0.91; p=0.04), respectively, with CRT alone in locally advanced cervical cancer (LBA8). The study involved 500 patients with stage IB1/2 to IVA, mainly squamous cell cervical carcinoma who had a median age of 46 years. Altogether, 77% had stage II disease and more than half (58%) were lymph node negative. Grade ≥3 adverse events were seen in 59% of patients receiving induction chemotherapy plus CRT and in 48% of patients receiving CRT alone. Median follow-up was 64 months.
Most (92%) patients in the induction chemotherapy arm received at least 5 cycles of carboplatin plus paclitaxel and the median interval between induction chemotherapy and CRT was 7 days. Over 80% of patients in each arm received at least 4 cycles of cisplatin as part of CRT (85% in the induction chemotherapy plus CRT arm and 90% in the CRT arm). External beam radiotherapy and brachytherapy were received by 97% and 98% of patients, respectively, in the induction chemotherapy plus CRT arm and by 92% and 97%, respectively, in the CRT arm. The median overall treatment time for CRT was 45 days in each arm.
“The results are encouraging for a disease that for several decades has failed to show improvements in long-term outcomes beyond those achieved with CRT alone and that has a high unmet need for new treatments,” says Prof. Ana Oaknin from Hospital Universitari Vall d'Hebron, Barcelona, Spain. “However, it is important to consider the population recruited and the large proportion of patients – 58% – who had node-negative disease, as we know that positive lymph nodes are indicative of a high-risk of relapse (Am J Clin Oncol 2009;32:411–416). Further analysis, in terms of nodal status, would be useful in determining the suitability of the induction chemotherapy approach for different relapse risk groups.” Oaknin notes the differences between the GCIG INTERLACE population and the high-risk population included in the positive phase III ENGOT-CX11/GOG-3047/KEYNOTE-A18 study investigating pembrolizumab plus CRT in locally advanced cervical cancer – presented at ESMO Congress 2023 – which stipulated that patients must have stage IB2–IIB node-positive or stage III–IVA disease. She also points out that while efforts were made to enrol from diverse health care settings over the 10-year completion period, 76% of the population were recruited from the UK.
Oaknin concludes: “Ongoing and planned trials may help to provide information on other ways to improve outcomes beyond CRT alone in high-risk, locally advanced cervical cancer. These include two maintenance therapy trials, the phase II ATOMICC trial (NCT03833479) investigating anti-PD1 therapy with dostarlimab and the phase III e-VOLVE Cervical Study (GOG-3092/ENGOT-cx19) trial looking at the use of the monovalent bispecific human IgG1 monoclonal antibody, volrustomig.”
Abstract discussed:
McCormack M, et al. A randomised phase III trial of induction chemotherapy followed by chemoradiation compared with chemoradiation alone in locally advanced cervical cancer. The GCIG INTERLACE trial. ESMO Congress 2023, LBA8
Presidential Symposium 2, 22.10.2023, h. 16:30 – 18:15, Madrid Auditorium – Hall 6