Despite positive results from trials, the use of different assays with variable sensitivities and specificities impact their interpretation and clinical validation
Liquid biopsy continues to consolidate its role as a tool for diagnosing solid tumours and monitoring treatment response, as some studies presented at the ESMO Congress 2023 (Madrid, 20–24 October) show. However, the large number of circulating tumour DNA (ctDNA) assays available and adopted in clinical research still represent a limiting factor to the validation of best approaches and the achievement of more solid evidence in the field.
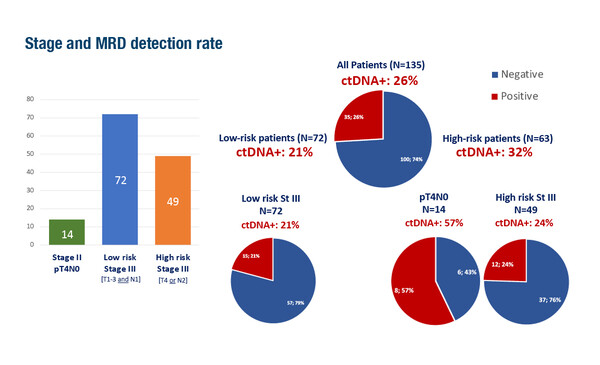
In a multicentre, prospective trial, the PEGASUS study, ctDNA analysis using a commercially available assay (Guardant Reveal) was used to examine minimal residual disease (MRD) after surgery and after adjuvant treatment in patients with stage III or high-risk stage II colon cancer, showing that liquid biopsy could help guide the post-surgical management of these patients by reducing unnecessary toxicity and improving response to chemotherapy (LBA28).
In the trial, post-surgical treatment included 3-month capecitabine–oxaliplatin if ctDNA+ or 6-month capecitabine treatment if ctDNA–, while in the post-adjuvant setting, patients were switched to folinic acid–fluorouracil–irinotecan (FOLFIRI) if ctDNA+, de-escalated to capecitabine if ctDNA– after capecitabine, or escalated to capecitabine–oxaliplatin if ctDNA+. Among 135 patients included in the per-protocol population, 35 (26%) patients were ctDNA+ after surgery. After 3-month capecitabine–oxaliplatin, 11/35 (31%) patients were converted to ctDNA–, but 8/11 (73%) eventually relapsed or became ctDNA+ again. Of 24 patients who received FOLFIRI after capecitabine–oxaliplatin, 13 (54%) remained ctDNA+ (6 of whom relapsed), and the remaining 11 (46%) were converted to ctDNA– and continued to be relapse free at the time of analysis (median follow-up 21.2 months).
“ctDNA clearance measured by the Guardant Reveal assay after standard 3-month capecitabine–oxaliplatin therapy does not appear to be indicative of treatment efficacy in eradicating MRD, given the high (73%) rate of subsequent relapse,” says Dr Jeanne Tie from the Peter MacCallum Cancer Centre, Melbourne and the Walter and Eliza Hall Institute of Medical Research, Parkville, Australia. “Almost half (11/24) of the patients who remained ctDNA+ after standard 3-month capecitabine–oxaliplatin cleared their ctDNA with ‘second-line’ adjuvant FOLFIRI, and remained recurrence-free at last follow-up. This is the first reported evidence that further therapy – in this case FOLFIRI – may be able to salvage MRD that is resistant to oxaliplatin-based adjuvant treatment.” She notes that another interesting finding from PEGASUS is the low relapse rate (7%) in patients with stage III and high-risk stage II colon cancer, despite only receiving single-agent capecitabine, supporting a de-escalation treatment strategy in low-risk ctDNA– patients.
Commenting on the methodology, Dr Umberto Malapelle from the University of Naples Federico II, Italy, says that studies such as PEGASUS are highly useful not only to define the analytical validity of this approach, but also to confirm its clinical validation. “However, we must consider that a gold standard is very difficult to define in this setting. In the absence of comparator tissue samples to test, positive methylation status in blood could be a false-positive, and the only option – the current gold standard – is clinical follow-up,” he highlights.
Other presentations at the Congress also point to the value of ctDNA analysis in the solid tumour setting. In patients with colorectal cancer (CRC), data from the PRECISION study showed that pre-treatment genomic alterations (detected using the Guardant360 assay) and 4-week post-operative MRD analysis (evaluated using the Guardant Reveal assay) were able to accurately stratify patients into prognostic categories and could be useful for guiding personalised adjuvant chemotherapy for those with resectable metastatic disease (Abstract 591P). An update of the GALAXY study in 2,176 patients with resected CRC confirmed that serial ctDNA status analysed using a commercial tumour-informed assay (Signatera) was the most significant prognostic factor; it was predictive of patient outcomes and could potentially be used to guide adjuvant chemotherapy (Abstract 558MO). Data from the phase III ASCOLT trial showed that tumour-naïve, serial ctDNA detection using a commercial assay (SafeSEQ) within 1 year of adjuvant chemotherapy was associated with recurrence in patients with resected CRC (Abstract 586P). Tie says, “These results confirm that ctDNA detection at the post-surgical and post-treatment landmark timepoint is an independent prognostic factor using both tumour-informed (GALAXY study) and tumour-agnostic assays (PEGASUS, PRECISION and ASCOLT trials) for curatively resected CRC, despite treatment with adjuvant capecitabine–oxaliplatin, as in the PEGASUS study.”
Malapelle cautions that while analysis of ctDNA using commercial and in-house laboratory assays offers the opportunity to examine the presence of various disease-associated mutations simultaneously, a key aspect to consider is the handling of the liquid biopsy sample. “We must consider the limited half-life of ctDNA – in many cases only 50 minutes or less – which impacts the optimal timing of sample collection. Also, different assays have different sensitivities and specificities, which impact the interpretation of results. For instance, the 14-gene MRD assay for CRC used in the ASCOLT trial had a sensitivity of 54% and a specificity of 86%, which are considered relatively low, but reflect the reality in the setting of resected, non-metastatic CRC. Ideally, assays should achieve sensitivities and specificities of ≥90%, but for stage I–III CRC, this is difficult to achieve with current assays because of the low quantity of ctDNA in liquid biopsy. However, an improvement in detection limit could increase the risk of false-positive results, for which we need appropriate biological controls.”
Despite the advances in the field, challenges remain to the imminent incorporation of liquid biopsy into the management of solid tumours in clinical practice. “High-level evidence is needed, preferably from randomised clinical trials exploring different management scenarios based on ctDNA results for each disease stage across a number of tumour types, as well as a greater understanding of the biological basis for false-negative results,” says Tie. Cost-effectiveness analyses are also a requirement to secure reimbursement of an assay in the healthcare systems of many countries. There may also be technical limitations preventing use of this technology in clinical practice. “The high-grade technology required to perform the assays is not uniformly available worldwide, and we need to democratise its access so that all patients benefit from it,” concludes Malapelle.
Abstracts discussed:
Lonardi S. The PEGASUS trial: post-surgical liquid biopsy-guided treatment of stage III and high-risk stage II colon cancer patients. ESMO Congress 2023, LBA28
Proffered Paper Session – Gastrointestinal tumours, lower digestive, 23.10.2023, h. 08.30 – 10.00, Barcelona Auditorium – Hall 9
Kobayashi S, et al. Survival and benefit of adjuvant chemotherapy (ACT) by circulating tumor DNA (ctDNA)-based genomic profile and molecular residual disease (MRD) in resectable colorectal oligometastases (CRM): PRECISION, a prospective multicenter study. ESMO Congress 2023, Abstract 591P
Poster Display Session – Colorectal cancer, 22.10.2023, h. 12.00 – 13.00, Hall 8
Nakamura Y, et al. Circulating tumor (ct)DNA as a prognostic biomarker in patients (pts) with resected colorectal cancer (CRC): an updated 24 months (mos) disease free survival (DFS) analysis from GALAXY study (CIRCULATE-JAPAN). ESMO Congress 2023, Abstract 558MO
Mini Oral Session – Gastrointestinal tumours, lower digestive, 22.10.2023, h. 14.45 – 16.20, Burgos Auditorium – Hall 3
Day D, et al. Minimal residual disease (MRD) detection using a tumour naïve circulating tumour DNS (ctDNA) assay in patients (pts) with resected colorectal cancer (CRC) in the phase III ASCOLT trial. ESMO Congress 2023, Abstract 586P
Poster Display Session – Colorectal cancer, 22.10.2023, h. 12.00 – 13.00, Hall 8