Final results from the RADICALS-RT trial show that observation with salvage RT for prostate-specific antigen failure should be the current standard strategy after surgery
According to the final results from the RADICALS-RT trial, presented at the ESMO Congress 2023 (Madrid, 20–24 October), adjuvant radiotherapy (RT) for patients at increased risk of cancer relapse following radical prostatectomy failed to improve key clinical endpoints, but induced significant side-effects when compared to the initiation of early salvage RT in cases of biochemical progression.
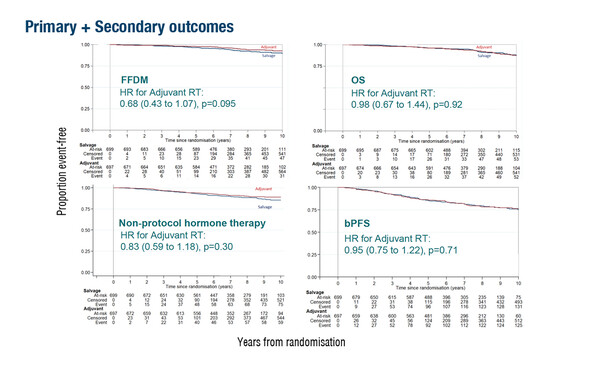
No difference in the 10-year freedom from distant metastases (FFDM) rate between adjuvant RT and observation plus salvage RT was reported in 1,396 patients undergoing radical prostatectomy (RP) who had post-operative prostate-specific antigen (PSA) ≤0.2 ng/mL and ≥1 risk factor (Abstract 1764O).The primary outcome data from the phase III RADICALS-RT trial, presented at the ESMO Congress 2023 (Madrid, 20–24 October), confirm initial results that did not support routine administration of adjuvant RT after surgery (Lancet. 2020;396:1413–1421).
With 80 events, the 10-year FFDM rate was 93% for adjuvant RT versus 90% in the early salvage RT group (hazard ratio [HR] 0.68; 95% confidence interval [CI] 0.43–1.07; p=0.095). Corresponding 10-year overall survival rates were 88% and 87% (HR 0.98; 95% CI 0.67–1.44; p=0.92). At 1 year, self-reported urinary incontinence and faecal incontinence were significantly worse with adjuvant RT (p≤0.001). A considerable proportion of patients in the early salvage RT group, around 60%, had not yet needed RT.
The RADICALS-RT trial is the largest study to have investigated adjuvant RT in prostate cancer. The median age of patients was 65 years and 37% had a CAPRA-S score of 6+. Patients with post-operative PSA ≤0.2 ng/mL and ≥1 risk factor (pT3/4, Gleason score 7–10, positive margins or pre-operative PSA ≥10 ng/mL) were randomised 1:1 up to 22 weeks after RP to receive adjuvant RT or observation plus salvage RT at biochemical relapse(two consecutive rises with a PSA ≥0.1 ng/mL or three consecutive rises). Stratification factors were Gleason score, margin status, planned RT volume (prostate bed alone versus prostate bed plus pelvis), RT schedule (52.5 Gy/20 fractions versus 66 Gy/33 fractions) and centre.
Abstract discussed:
Parker C, et al. Timing of radiotherapy (RT) after radical prostatectomy (RP): Final results of RADICALS RT randomised controlled trial [NCT00541047]. ESMO Congress 2023, Abstract 1764O
Proffered Paper Session – Genitourinary tumours, prostate, 20.10.2023, h. 16:00 – 17:20, Granada Auditorium – Hall 3