Results from the JCOG1611-GENERATE trial fuel the debate around the preferred option in this setting
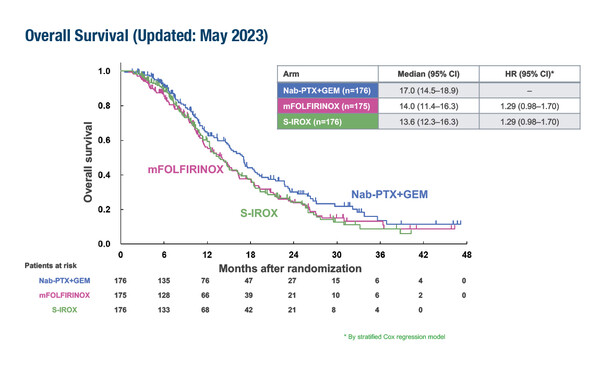
As presented at the ESMO Congress 2023 (Madrid, 20–24 October), a planned interim analysis on 527 patients in the multicentre, randomised, open-label, phase II/III JCOG1611-GENERATE trial supports the use of nab-paclitaxel plus gemcitabine as first-line treatment for patients with metastatic or recurrent pancreatic cancer compared with modified FOLFIRINOX (oxaliplatin 85 mg/m2, irinotecan 150 mg/m2, l-leucovorin 200 mg/m2 and fluorouracil 2,400 mg/m2 on days 1–3, every 2 weeks) or S-IROX (oxaliplatin 85 mg/m2, irinotecan 150 mg/m2 and S-1 80 mg/m2/day on days 1–7, every 2 weeks) (Abstract 1616O).
As Dr Angela Lamarca highlights in her editorial, these findings open a very interesting debate about which therapy regimen may be preferable in this setting.
Investigators reported median overall survival times of 17.0 months with nab-paclitaxel (125 mg/m2) plus gemcitabine (1,000 mg/m2), 14.0 months with modified FOLFIRINOX (hazard ratio [HR]1.29; 95% confidence interval [CI] 0.98–1.70) and 13.6 months with S-IROX (HR 1.29; 95% CI 0.98–1.70) in May 2023. These updated data were similar to those from the planned analysis in March 2023 where the predictive probability for achieving superiority at the final analysis was calculated as 0.73% for the modified FOLFIRINOX arm and 0.48% for the S-IROX arm, leading to termination of the trial for futility. Both triplet therapies were also associated with a higher incidence of the most common grade ≥3 non-haematological toxicity (anorexia), with rates of 22.8% and 27.6% in the modified FOLFIRINOX and S-IROX arms, respectively, compared with 5.2% in the nab-paclitaxel plus gemcitabine arm.
The trial enrolled patients aged between 20–75 years with pathologically confirmed metastatic or recurrent pancreatic cancer and an Eastern Cooperative Oncology Group performance status of 0 or 1, who were randomised 1:1:1 to treatment at centres in Japan.
Abstract discussed:
Ohba A, et al. Nab-paclitaxel plus gemcitabine versus modified FOLFIRINOX or S-IROX in metastatic or recurrent pancreatic cancer (JCOG1611, GENERATE): a multicenter, randomized, open-label, three-arm, phase 2/3 trial. ESMO Congress 2023, Abstract 1616O
Proffered Paper Session 2 – Gastrointestinal tumours, upper digestive, 22.10.2023, h. 10:15 – 11:45, Barcelona Auditorium – Hall 9