Findings from the SANO trial also reported clinically complete responses after neoadjuvant chemoradiotherapy
Active surveillance after neoadjuvant chemoradiotherapy may be an alternative to surgery for some patients with oesophageal cancer, as suggested by encouraging data from the SANO trial presented at the ESMO Congress 2023 (Madrid, 20–24 October) (LBA75).
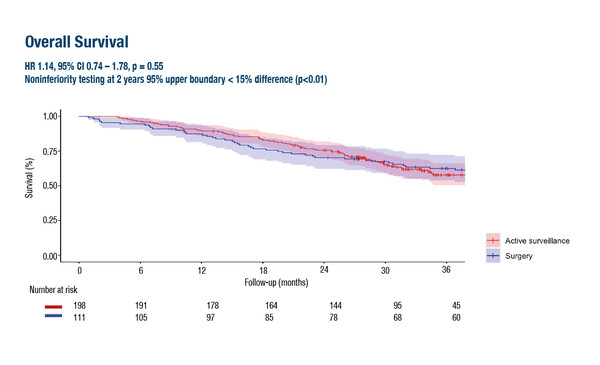
Findings revealed that overall survival (OS) from the day of clinically complete response (CCR) – the primary endpoint – was not inferior to surgery at 2 years in patients with oesophageal cancer who underwent active surveillance (hazard ratio [HR] 1.14, 95% confidence interval [CI] 0.74–1.78; p=0.55). In addition, global health-related quality of life (HRQoL) using the EORTC QLQ-C30 was significantly better at 6 and 9 months in patients who received active surveillance than surgery, with differences indicative of a medium effect (Cohen’s d >0.50).
The SANO trial was a noninferiority stepped-wedge cluster randomised trial in which patients with CCR (i.e. no residual disease 6 and 12 weeks after neoadjuvant chemoradiotherapy [nCRT]) underwent active surveillance (n=198) or standard surgery (n=111). In the active surveillance arm, surgical resection was offered only to patients in whom locoregional regrowth was highly suspected or proven, and in the absence of distant dissemination.
Prof. Magnus Nilsson of CLINTEC, Karolinska Institutet, and the Karolinska Hospital, Stockholm, Sweden, advises, “It is a dream for oncologists and surgeons to be able to cure oesophageal cancer without surgery in the future because surgery has significant lifelong HRQoL drawbacks. The data from SANO are really encouraging. Two previous trials addressed this around 15 years ago (J Clin Oncol. 2007;25:1160–1168; J Clin Oncol. 2005;23:2310–2317), but were premature in terms of available treatment techniques and clinical response evaluation accuracy, and therefore failed to change practice.”
In the SANO trial and previous trials, both histological oesophageal cancer types have been assessed together – squamous cell carcinoma (SCC), which comprises almost 90% of incident cases globally but only 20% in Western Europe, and adenocarcinoma, the dominant type in Western countries but not globally (Gastroenterology. 2018;154:360–373; Gut. 2015;64:381–387). “These are biologically very different, with SCC having a much better response to CRT than adenocarcinoma,” notes Nilsson. “Unfortunately, we do not yet have access to data from the SANO trial stratified by histological type, but this will be crucial to the conclusions that can be drawn. We are much closer to reaching non-surgical cure for SCC versus adenocarcinoma. For adenocarcinoma, I suspect we will need better systemic biomarker-driven treatment and we are now rapidly getting more options for this.”
According to Nilsson, the majority of patients who do not achieve CCR should also be taken into account, referring to recent published data from the NeoRes II trial showing a detriment to clinical outcomes in delaying surgery by 10–12 weeks compared to 4–6 weeks after completed nCRT (Ann Oncol. 2023 Aug 30:S0923-7534(23)00825-6). He concludes, “Many patients in the large group of non-complete responders, who were not included in the cluster-randomised comparison, are likely to have been operated on at ≥10 weeks after completing nCRT. Before we can apply the SANO-algorithm broadly in clinical practice, we also need to assess the outcome in these patients. We may actually need a new randomised trial comparing all patients upfront entering the SANO algorithm versus the current standard of care in which surgery is performed within 6 weeks. Both QoL and OS will be important endpoints.”
Abstracts discussed:
Van der Wilk BJ, et al. Neoadjuvant chemoradiotherapy followed by surgery versus active surveillance for oesophageal cancer (SANO-trial): a phase-III stepped-wedge cluster randomised trial. ESMO Congress 2023, LBA75
Proffered Paper Session 1 – Gastrointestinal tumours, upper digestive, 20.10.2023, h. 14:00 – 15:40, Barcelona Auditorium – Hall 9