Neoadjuvant, perioperative, targeted and combined treatment approaches have shown potential benefit for patients with this aggressive disease, but further research is needed
Pancreatic cancer, notorious for its aggressiveness and poor prognosis, remains an area of highest unmet medical need and one of the biggest challenges in modern oncology. Four clinical trials presented at the ESMO Congress 2023 (Madrid, 20–24 October) explore different innovative strategies to improve treatment of patients with pancreatic cancer.
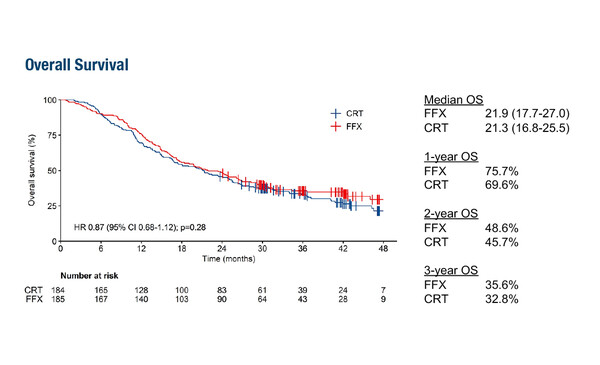
In a first study, the phase III PREOPANC-2 trial, data showed no overall (OS) benefit – the primary endpoint – of neoadjuvant FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy in patients with borderline resectable and resectable pancreatic adenocarcinoma (PDAC; LBA83). Median OS was 21.9% versus 21.3%, respectively (hazard ratio 0.87; 95% confidence interval [CI] 0.68–1.12; p=0.28). Resection rates (77% versus 75%, respectively; p=0.7) and serious adverse rates (49% versus 43%, respectively; p=0.26) were also similar between treatment arms.
The previous PREOPANC trial (J Clin Oncol. 2022;40:1220–1230) showed improved OS with neoadjuvant gemcitabine-based chemoradiotherapy followed by surgery and adjuvant gemcitabine versus upfront surgery and adjuvant gemcitabine in resectable and borderline resectable pancreatic cancer. Dr Benedikt Westphalen from the University Hospital Munich, Germany, notes, “The PREOPANC-2 trial hypothesised that FOLFIRINOX might supersede the efficacy of gemcitabine-based chemoradiotherapy in the neoadjuvant setting, but has concluded otherwise. Median OS was almost congruent between treatment arms, and resection and serious adverse event rates were also similar. Further work is therefore needed to explore options for the management and neoadjuvant treatment of borderline, locally advanced and resectable pancreatic cancer.”
A second study, the single-arm phase II nITRO study, found perioperative NALIRIFOX was manageable and active in 107 patients with resectable PDAC (Abstract 1620P). Overall, 87 patients underwent surgical exploration and a 65.3% R0 resection rate was achieved, exceeding the alternative hypothesis of 55%. After a median follow-up of 33.1 months, median OS in the intention-to-treat population was 32.3 months (95% CI 27.8–44.3) and resected patients achieved a median OS of 44.3 months (95% CI 33.2–not assessable). TNF-α was the circulating factor that correlated most significantly with response and survival outcomes. Westphalen comments, “In the treatment of resectable PDAC, the role of neoadjuvant and perioperative strategies lies at the core of a fierce scientific debate. The authors conclude that NALIRIFOX could be a potentially viable and effective perioperative regimen. The correlative findings between TNF-α, a cytokine pivotal in chemoresistance, and survival outcomes support the approach of targeting molecular pathways in fine-tuning therapeutic strategies for resectable PDAC.”
Positive data for the role of precision medicine in pancreatic cancer were presented from the phase II RAGNAR trial (Abstract 1621P), showing clinically meaningful activity of erdafitinib (an FGFR inhibitor) in 18 patients with previously treated advanced/metastatic pancreatic cancer and prespecified FGFR1–4 alterations (14 FGFR2, 4 FGFR1). The overall response rate (ORR) was 55.6% (95% CI 30.8–78.5) and median OS was 19.7 months. Safety data were consistent with the known profile of erdafitinib. According to Westphalen, “The noteworthy ORR and OS data from this study demonstrate potent clinical activity, albeit in a rather select patient population. The study introduces a discerning approach in treating pancreatic cancer by tailoring interventions based on specific genetic alterations.”
Finally, preliminary results from another phase II study (n=36) showed clinically meaningful improvement and tolerability using a tri-combination strategy of CBP501, cisplatin and nivolumab as third-line treatment in patients with metastatic PDAC (Abstract 1625P). Three-month progression-free survival (PFS) rates were 44% (CBP501 25 mg/m2 with cisplatin and nivolumab), 22% (CBP501 16 mg/m2 with cisplatin and nivolumab), 11% (CBP501 25 mg/m2 with cisplatin) and 33% (cisplatin and nivolumab). ORR was 22% with the triple combination (2 partial responses), but 0% in the other arms. “Based on PFS and ORR, this trial with CBP501, cisplatin and nivolumab demonstrates a potential role in the third-line setting,” notes Westphalen. “Although the outcomes accentuate the necessity for subsequent lines of treatment, the trajectory towards amalgamating chemotherapy and immunotherapy warrants further refinement in both stratifying patients and optimising regimens to improve outcomes.”
On a holistic note, Westphalen concludes, “A collective scrutiny of these studies not only underscores the potential of neoadjuvant, perioperative, targeted and combined treatment approaches, but also illuminates the arduous path ahead in unravelling optimal therapeutic solutions for pancreatic cancer. The PREOPANC-2 trial summons the oncology community to probe deeper into formulating and refining neoadjuvant regimens, while the promising results from nITRO and RAGNAR elucidate how personalised and targeted approaches could possibly tailor better outcomes for subgroups of patients. Meanwhile, exploring novel combinations as revealed for CBP501, cisplatin and nivolumab may open new horizons for managing advanced-stage patients.”
Abstracts discussed:
Koerkamp BG, et al. Neoadjuvant chemotherapy with FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy for borderline resectable and resectable pancreatic cancer (PREOPANC-2): a multicenter randomized controlled trial. ESMO Congress 2023, LBA83
Proffered Paper Session 2 – Gastrointestinal tumours, upper digestive, 22.10.2023, h. 10:15 – 11:45, Barcelona Auditorium – Hall 9
Melisi D, et al. A phase 2 study of perioperative NALIRIFOX in patients with resectable pancreatic ductal adenocarcinoma (rPDAC): survival update and biomarkers analysis of the nITRO trial. ESMO Congress 2023, Abstract 1620P
Poster Display – Pancreatic cancer, 23.10.2023, h. 12:00 – 13:00, Hall 8
Pant S, et al. Efficacy and safety of erdafitinib in adults with pancreatic cancer and prespecified fibroblast growth factor receptor alterations (FGFRalt) in the phase 2 open-label, single-arm RAGNAR trial. ESMO Congress 2023, Abstract 1621P
Poster Display – Pancreatic cancer, 23.10.2023, h. 12:00 – 13:00, Hall 8
Enzler T, et al. Multicenter, randomized, parallel group, phase 2 study to establish the efficacy and safety of CBP501, cisplatin, and nivolumab for ≥3rd line treatment of patients with exocrine pancreatic cancer and WBC. ESMO Congress 2023, Abstract 1625P
Poster Display – Pancreatic cancer, 23.10.2023, h. 12:00 – 13:00, Hall 8