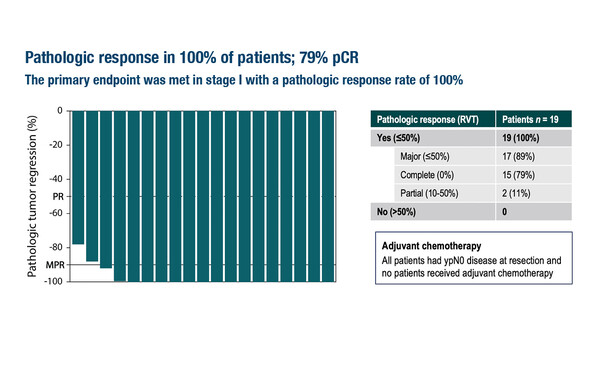
In the NICHE-3 study, all patients achieved a pathological response
According to results presented at the ESMO Congress 2023 (Madrid, 20–24 October), neoadjuvant nivolumab plus relatlimab led to a pathological complete response rate of 79%, a major pathological response rate of 89% and an overall pathological response rate of 100% among 19 patients with resectable, locally advanced, MMR-deficient (dMMR) colon cancer (LBA31). With a median interval of 7.4 weeks between the first dose of treatment and surgical resection, all patients underwent surgery without delay.
Altogether, 74% of patients experienced grade 1–2 immune-related adverse events (irAEs), most commonly infusion-related reactions (32%). Only 1 patient experienced a grade 3 irAE (hyperthyroidism). Four patients had endocrinopathy for which supplementation was required; one of these patients had hypothyroidism and the other three had hypophysitis with secondary adrenal insufficiency. There were no grade 4–5 irAEs.
The NICHE-3 study is the first to demonstrate the efficacy and safety of the nivolumab/relatlimab combination in dMMR colon cancer. It is built on positive findings from two previous studies – the NICHE-2 study, which showed the efficacy of neoadjuvant nivolumab/ipilimumab in dMMR colon cancers and which was reported at the ESMO Congress 2022, and a phase II study demonstrating the promising activity of neoadjuvant nivolumab/relatlimab in melanoma (Nature. 2022;611:155–160). In addition, the anti-PD-1 inhibitor dostarlimab has demonstrated an impressive rate of complete responses in patients with dMMR rectal cancer (N Engl J Med. 2022;386:2363–2376). Having attained the pre-specified response criterion of ≥15/19 patients, accrual in NICHE-3 is continuing.
Abstract discussed:
Verschoor YL, et al. Neoadjuvant nivolumab plus relatlimab (anti-LAG3) in locally advanced MMR-deficient colon cancers: the NICHE-3 study. ESMO Congress 2023, LBA31
Mini Oral Session – Gastrointestinal tumours, lower digestive, 22.10.2023, h. 14:45 – 16:20, Burgos Auditorium – Hall 3