The impact of immunotherapy on disease progression and survival remains uncertain, but biomarker selection may be the future
Interim analyses from two phase III trials investigating the addition of immunotherapy to chemotherapy in the perioperative setting for patients with biomarker unselected, untreated, resectable gastric/gastro-oesophageal junction (GC/GEJ) cancers show positive findings for pathological complete response (pCR) rate, but their impact on survival is still uncertain.
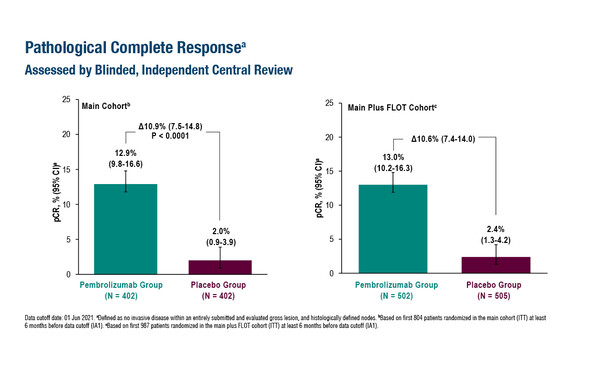
As presented at the ESMO Congress 2023 (Madrid, 20–24 October), data from the KEYNOTE-585 study (third interim analysis) reported an improved pCR rate (primary endpoint) in the first 987 patients randomised to the PD-1 inhibitor pembrolizumab compared with placebo added to neoadjuvant and adjuvant treatment with chemotherapy (fluorouracil plus cisplatin [FP] or fluorouracil, leucovorin, oxaliplatin plus docetaxel [FLOT]) (13.0% versus 2.4%; treatment difference 10.6% (95% confidence interval [CI] 7.4–14.0; p<0.0001) (LBA74). Most patients in the main cohort (n=804) did not receive FLOT, and pCR rates were 12.9% with pembrolizumab plus chemotherapy versus 2.0% with placebo plus chemotherapy (treatment difference 10.9%; 95% CI 7.5–14.8; p<0.00001).
Combination therapy did not significantly prolong the co-primary endpoint of event-free survival (EFS) in either the main cohort (median 44.4 months versus 25.3 months; HR 0.81; 95% CI 0.67–0.99; p=0.0198) or the main plus FLOT cohort (median 45.8 months versus 25.7 months; HR 0.81; 95% CI 0.68–0.97). Median overall survival in the main cohort (a further primary endpoint) was 60.7 months with pembrolizumab and 58.0 months with placebo (HR 0.90; 95% CI 0.73–1.12) and continues to be monitored until the final analysis. Rates of grade ≥3 drug-related adverse events (AEs) were similar with pembrolizumab and placebo in the main cohort (65% versus 63%, respectively).
“On the one hand it is perhaps surprising that the improvement in pCR seen in KEYNOTE-585 did not lead to prolonged survival because we know that, in chemotherapy trials, regimens associated with higher pCR rates tend to improve survival,” says Dr Elizabeth Smyth from the Oxford University Hospitals NHS Foundation Trust, UK, “but on the other hand, we know that immunotherapy in gastric cancer performs best in patients with PD-L1-positive disease and that patients in this trial were biomarker unselected. For patients with tumours that express high levels of PD-L1 (combined positive score ≥10), the difference in EFS for those treated with pembrolizumab was meaningful (HR 0.70).” She continues: “Another factor to consider is that most of the patients received cisplatin-based chemotherapy rather than the standard of care, FLOT, the oxaliplatin element of which is thought to be more active in combination with immune checkpoint inhibitors (ICIs) than cisplatin.”
The second of the two trials presented was the MATTERHORN trial, which reported a significant increase in the pCR rate among patients randomised to durvalumab plus FLOT (n=474) compared with patients randomised to placebo plus FLOT (n=474) (19% versus 7%, respectively; treatment difference 12%; odds ratio 3.08, 95% CI 2.03–4.67; p<0.00001) (LBA73). The combined pCR/near-complete pathological response rate was 27% for durvalumab versus 14% with placebo. There was no difference between durvalumab and placebo in the proportion of patients completing surgery (87% versus 84%) or the rate of R0 resection (86% in each arm). Among patients who underwent surgery, downstaging favoured durvalumab (pT0, 23% with durvalumab versus 11% with placebo; pN0, 52% versus 36%; by central review). The rates of grade 3–4 AEs (69% with durvalumab versus 68% with placebo), treatment-related AEs (TRAEs; 95% versus 94%) and grade 3–4 TRAEs (58% versus 56%) were similar between treatment arms. The study is ongoing for the primary endpoint of EFS.
“The MATTERHORN trial is essentially the same design as the KEYNOTE-585 trial, but the use of FLOT – which is the ESMO Guidelines recommended standard of care – as the only chemotherapy regimen is reflected in the higher pCR rates achieved, even in the control arm, compared with the KEYNOTE-585 trial,” says Smyth. “It is particularly encouraging to note the safety of the combination of durvalumab plus FLOT and that it did not compromise the potential for patients to undergo surgery. However, while the results are promising, the EFS data are required before any impact on clinical practice can be suggested.”
The results from these trials were eagerly awaited by oncologists due to an approximate 50% relapse rate currently observed in patients with GC/GEJ cancers undergoing surgical resection following FLOT (Lancet. 2019;393:1948–1957). However, Smyth concludes, “These findings are insufficient to recommend ICIs in perioperative therapy. If the EFS results from the MATTERHORN trial are not positive, we will need to proceed to biomarker-selected trials. As well as the obvious candidate, PD-L1, microsatellite instability-high (MSI-H) tumours, which make up to 8–10% of operable gastroesophageal adenocarcinoma, also show excellent sensitivity to immunotherapy and trials are investigating combination ICI treatments in MSI-H subgroups.”
Abstracts discussed:
Shitara K, et al. Pembrolizumab plus chemotherapy vs chemotherapy as neoadjuvant and adjuvant therapy in locally-advanced gastric and gastroesophageal junction cancer: the phase 3 KEYNOTE-585 study. ESMO Congress 2023, LBA74
Proffered Paper Session 1 – Gastrointestinal tumours, upper digestive, 20.10.2023, h. 14:00 – 15:40, Barcelona Auditorium – Hall 9
Janjigian YY, et al. Pathological complete response (pCR) to durvalumab plus 5-fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) in resectable gastric and gastroesophageal junction cancer (GC/GEJC): interim results of the global, phase 3 MATTERHORN study. ESMO Congress 2023, LBA73
Proffered Paper Session 1 – Gastrointestinal tumours, upper digestive, 20.10.2023, h. 14:00 – 15:40, Barcelona Auditorium – Hall 9