Encouraging efficacy data with anti-PD1 antibody in combination or as monotherapy
Two Late-Breaking Abstract presentations at ESMO Congress 2022 showed encouraging efficacy data from phase III trials of first-line treatment with an anti-PD1 antibody with or without a kinase inhibitor in patients with advanced hepatocellular carcinoma (HCC).
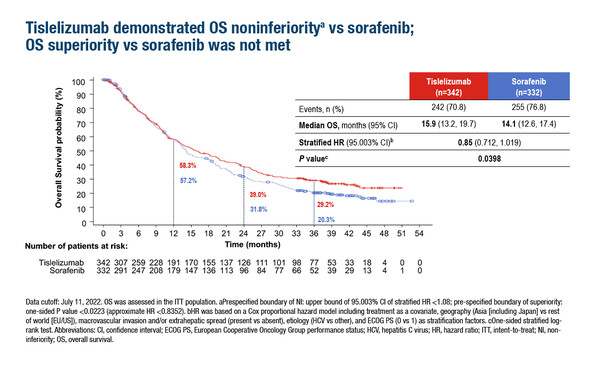
The phase III open-label RATIONALE-301 trial evaluated the non-inferiority of the anti PD-1 antibody tislelizumab (200 mg IV every 3 weeks) versus sorafenib (400 mg orally twice daily), a kinase inhibitor, in patients with unresectable HCC (LBA36). The primary endpoint of overall survival (OS) was met (median 15.9 months with tislelizumab versus 14.1 months with sorafenib; hazard ratio [HR] 0.85; 95% confidence interval [CI] 0.712–1.019; p=0.0398).
Positive results were also reported in a phase III trial of the anti-PD-1 antibody camrelizumab (200 mg IV every 2 weeks) plus the TKI rivoceranib (apatinib; 250 mg orally daily) versus sorafenib (400 mg orally twice daily) (LBA35). Significant improvements in progression-free survival (PFS; 5.6 months with camrelizumab plus rivoceranib versus 3.7 months with sorafenib; HR 0.52; 95% CI 0.41–0.65; p<0.0001) and median OS (22.1 months versus 15.2 months; HR 0.62; 95% CI 0.49–0.80; p<0.0001) were reported.
“The findings from these two trials could potentially be practice changing in advanced HCC,” comments Prof. Stephen Chan from the Chinese University of Hong Kong. “The RATIONALE-301 trial is a phase III trial demonstrating the non-inferiority of first-line tislelizumab and sorafenib. Tislelizumab is generally more tolerable than sorafenib so these results are meaningful in clinics. Similarly, the improvements in PFS and OS seen with first-line camrelizumab plus rivoceranib versus sorafenib are very encouraging. As expected, the toxicity of the combination is higher than with sorafenib monotherapy, but this is manageable.” Chan continues, “Although these two studies provide clinicians with more evidence to support the use of these regimens, we need more real-world data to confirm the findings in different populations.”
Negative results were reported for the phase III LEAP-002 study that evaluated first-line lenvatinib (8 or 12 mg/day depending on body weight) plus pembrolizumab (200 mg IV every 3 weeks) versus lenvatinib plus placebo in patients with advanced HCC (LBA34). After a median follow-up of 17.6 months for the final PFS and 32.1 months for the final OS, the primary endpoints of OS and PFS did not meet pre-specified statistical significance; median PFS was 8.2 months with lenvatinib plus pembrolizumab versus 8.0 months with lenvatinib (HR 0.867; 95% CI 0.734–1.024; p=0.0466), and median OS was 21.2 months versus 19.0 months, respectively (HR 0.840; 95% CI 0.708–0.997; p=0.0227). Grade 3–5 adverse events occurred in 62.5% of patients receiving lenvatinib plus pembrolizumab and 57.5% of patients receiving lenvatinib.
Chan comments, “These results are unexpected as the combination of lenvatinib plus pembrolizumab previously showed promising efficacy in a phase I trial (J Clin Oncol. 2020;38:2960–2970). Also, lenvatinib was approved for the first-line treatment of HCC based on the results of the REFLECT trial (Lancet. 2018;391:1163–1171). Notably, in the LEAP-002 trial, OS with lenvatinib monotherapy is the longest we have seen with a TKI – 19.0 months – which is much longer than the median OS of lenvatinib – 13.6 months – shown in the REFLECT trial. There may still be a future for this combination; we await with interest the results from an ongoing phase III LEAP-012 trial of lenvatinib plus pembrolizumab in patients with incurable, non-metastatic HCC.”
To conclude Chan notes, “These are exciting times in advanced HCC, but we need more research to allow us to move towards personalised treatment. We know which genes are dysregulated in HCC, but we are not yet translating or applying these findings to clinical practice. To advance the field and develop personalised treatment, we need to optimise treatment sequences, identify biomarkers and obtain biopsies before treatment so we can correlate treatment outcomes with the molecular signature of the tumour.” Chan goes on to stress, “A further key challenge in HCC is the under-representation in clinical trials of patients with poor liver function. In the future, we could also consider evaluating anti-PD1 antibody treatment for these patients.”
Abstracts presented:
Finn R, et al. Primary results from the phase 3 LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). ESMO Congress 2022, LBA34
Proffered Paper Session 1 – GI, upper digestive, 10.09.2022, h. 08:30 – 10:00, Cannes Auditorium
Kudo M, et al. Final analysis of RATIONALE-301: Randomized, phase 3 study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. ESMO Congress 2022, LBA36
Proffered Paper Session 1 – GI, upper digestive, 10.09.2022, h. 08:30 – 10:00, Cannes Auditorium
Qin S et al. Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): A randomized, phase III trial. ESMO Congress 2022, LBA35
Proffered Paper Session 1 – GI, upper digestive, 10.09.2022, h. 08:30 – 10:00, Cannes