Standard second-line chemotherapy may benefit fit patients but efficacy is limited and new treatments are urgently needed
Three studies presented at ESMO Congress 2022 focused on the role of second-line chemotherapy in advanced biliary tract cancer (BTC), identifying overall modest efficacy and highlighting the importance of tolerability and quality of life.
Data from the prospective, randomised, phase II NALIRICC study, presented in a Mini Oral Session, showed that the trial did not meet its primary endpoint of progression-free survival (PFS) improvement (Abstract 53MO). In a different presentation (Abstract 55P), the final analysis of the phase IIb randomised open-label NIFTY study in patients with metastatic BTC demonstrated a modest improvement in PFS for the combination of nano-liposomal irinotecan (nal-IRI) to 5-fluorouracil (5-FU)/leucovorin (LV) compared with 5-FU/LV alone as second-line treatment; these results were unexpected following the initial analysis of the NIFTY study, that reported a median PFS of above 7 months in the experimental arm (Lancet Oncol. 2021;22:1560–1572).
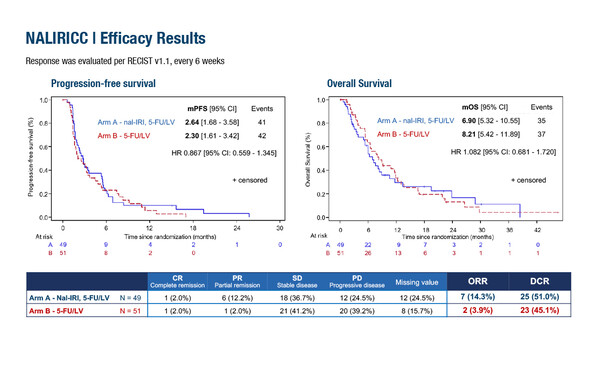
In the phase II NALIRICC study (Abstract 53MO), the addition of nal-IRI plus 5-FU/LV versus 5-FU/LV alone in the second-line treatment of patients with advanced BTC did not improve PFS (median 2.64 months versus 2.30 months, respectively) or overall survival (OS; median 6.90 months versus 8.21 months, respectively) and was associated with higher toxicity (grade ≥3 adverse events occurred in 70.8% versus 50% of patients, respectively). Objective response rate (ORR) was 14.3% in patients who received nal-IRI plus 5-FU/LV and 3.9% in those who received 5-FU/LV alone.
Similar activity data were identified in the final analysis of the NIFTY study (Abstract 55P). The combination improved PFS per blinded independent central review (BICR) (median 4.2 months [95% confidence interval (CI) 2.8–5.3] versus 1.7 months [95% CI 1.4–2.6], respectively; hazard ratio [HR] 0.61 [95% CI 0.44–0.86]; p=0.004), OS (median 8.6 months [95% CI 5.4–10.5] versus 5.3 months [95% CI 4.7–7.2], respectively; HR 0.68 [95% CI 0.48–0.95]; p=0.02), and ORR by BICR (12.5% versus 3.5%, respectively; p=0.043).
Prof. Robin Kate Kelley from the Helen Diller Family Comprehensive Cancer Center of the University of California, San Francisco, USA, notes that, “These two studies focusing on the role of nal-IRI combined with 5-FU/LV as second-line therapy for advanced stages of BTC had divergent results, with the NIFTY trial demonstrating modest improvement in PFS while the NALIRICC trial was negative, but they both underscore several common themes. Key differences between the trials were that the NIFTY trial enrolled an Asian population with a higher proportion of extrahepatic site of tumour than NALIRICC, and the fact that NIFTY trial used a lower dose of nal-IRI (70 mg/m2) than the 80 mg/m2 used in NALIRICC. These differences in population and treatment may have impacted on the primary PFS endpoint results. A common theme in both trials was the overall modest efficacy of the combination treatment arms, with median PFS of 2.64 months in NALIRICC and 4.2 months in NIFTY. Both studies demonstrated somewhat higher rates of ORR for the combination arms, with ORR 14.3% and 12.5% versus 3.9% and 3.5%, respectively. Collectively, the results reinforce the findings of prior retrospective studies of 5-FU/LV-based chemotherapy in the second-line context which have also reported PFS values in the 3-month range.” (Cancer. 2015;121:3290–3297; Acta Oncol. 2016;55:1168–1174; Lancet Oncol. 2021 May;22(5):690-701)
Kelley felt that another important finding from the final NIFTY analysis was the discordance between the original, blinded independent central review (BICR) PFS results and investigator review which had been presented in the initial published study report (Lancet Oncol. 2021;22:1560–1572): “At the time of the initial report, the median PFS as assessed by BICR for the combination arm was reported to be 7.1 months, while the investigator-assessed PFS was much shorter at 3.9 months; the PFS medians for the 5-FU/LV arms were 1.4 and 1.6 months, respectively. The updated data from the study analysed PFS utilising a different BICR team, which determined the median PFS to be 4.2 months, similar to the investigator-assessed median PFS (3.9 months). These updated results underscore the importance of capturing both BICR and investigator-assessed efficacy endpoints, particularly in anatomically complex diseases such as cholangiocarcinoma.” Kelley concludes that, “This pair of abstracts suggest only modest efficacy for the combination of nal-IRI plus 5-FU/LV, but a subset of patients who are fit for second-line therapy may derive benefit.”
Quality of life (QoL) data from the randomised phase III, multicentre, open-label ABC-06 trial of second-line active symptom control (ASC) versus ASC plus cisplatin/5-FU (ASC plus mFOLFOX) in patients with advanced biliary cancers also featured in a Mini Oral Session (Abstract 54MO). The data suggested treatment with ASC plus mFOLFOX was not detrimental on time to deterioration of Global Health Scale and that it may allow stabilisation of QoL scales and avoid worsening of nausea and pain at 4 months after baseline assessment, although further analyses are ongoing. Kelley considered that, “These data support the decision to recommend second-line therapy in fit patients, despite efficacy remaining below the threshold we hope to achieve. The QoL preservation seen in ABC-06 patients treated with mFOLFOX reinforces that modest efficacy benefits do translate to a real-world patient benefit.”
While some positive data are presented at ESMO Congress 2022 for patients with BTC, Kelley stresses that novel therapies are crucial. “New treatments for BTC are urgently needed as the efficacy of standard first-line treatment comprising gemcitabine plus cisplatin is limited with a median OS of less than a year (Cancer Manag Res. 2019;11:2623–2642). Standard chemotherapy also has limited efficacy in the second-line setting, with PFS around 4 months and response rates below 10% (Lancet Oncol. 2021;22:690–701)”.
Abstracts presented:
Vogel A, et al. Nal-IRI and 5-FU/LV compared to 5-FU/LV in patients with cholangio- and gallbladder carcinoma previously treated with gemcitabine-based therapies (NALIRICC – AIO-HEP-0116). ESMO Congress 2022, Abstract 53MO
Mini Oral Session, 10.09.2022, h. 14:45 – 16:15, Toulouse Auditorium
Yoo C, et al. Final results from the NIFTY trial, a phase IIb, randomized, open-label study of liposomal irinotecan (NAL-IRI) plus fluorouracil (5-FU)/leucovorin (LV) in patients (pts) with previously treated metastatic biliary tract cancer (BTC). ESMO Congress 2022, Abstract 55P
Poster display, Hall 4. An e-Poster is also available on the Congress virtual platform
Lamarca A, et al. Quality of life (QoL) and value of health (V-He) in advanced biliary cancers (ABC) treated with second-line active-symptom-control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC+FOLFOX) in the randomised phase III, multi-centre, open-label ABC-06 trial. ESMO Congress 2022, Abstract 54MO
Mini Oral Session, 10.09.2022, h. 14:45 – 16:15, Toulouse Auditorium