Two early studies investigate the anticancer activity of derazantinib and RLY-4008 in patients with FGFR inhibitor-naïve cholangiocarcinoma
Targeting FGFR has recently emerged as a promising strategy in the treatment of cholangiocarcinoma (CCA), a very aggressive rare malignancy. At ESMO Congress 2022, two new agents were added to the list of FGFR inhibitors being investigated, showing some clinical benefits or potential in patients not previously treated with this anticancer drug class.
Dr Chiara Braconi from the University of Glasgow, UK highlights the current challenges in CCA: “Despite showing remarkable activity, there are several issues with current FGFR inhibitors. These therapies are limited to a niche group of 10–15% of patients with CCA who have FGFR2 fusions and rearrangements; patients with CCA tumours that harbour other FGFR amplifications and mutations are not currently eligible to receive these treatments. Also, de novo FGFR2 mutations may emerge during treatment with FGFR inhibitors leading to secondary resistance to these agents.” Braconi cautions that adverse events related to FGFR-targeting agents is also a problem. “Side-effects that are specifically related to inhibition of FGFR, such as retinopathy and hyperphosphatemia may be problematic for some patients. Therefore, optimising use of these treatments is an important goal,” says Braconi.
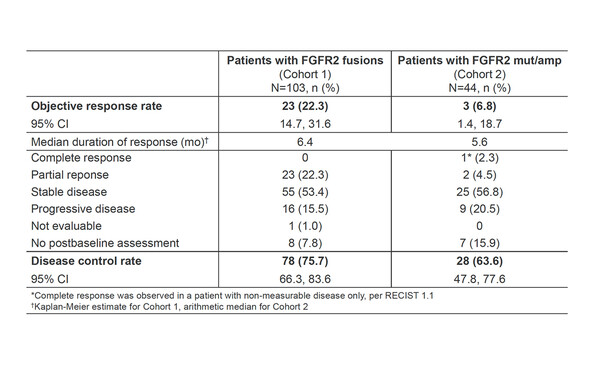
In the open-label phase II FIDES-01 study (NCT03230318), the anticancer activity of the FGFR1–3 kinase inhibitor, derazantinib, was evaluated in patients with inoperable or advanced intrahepatic CCA with FGFR2 fusions (FGFR2F; n=103) or mutations or amplifications (FGFR2MA; n=44 [n=31 response evaluable]) who received at least one prior chemotherapy (Abstract 59P). Results (data cutoff 25 March 2022) showed a confirmed objective response rate (ORR) of 22.3% (95% confidence interval [CI] 14.7–31.6) in patients with FGFR2F and 6.8% (95% CI 1.4–18.7) in patients with FGFR2MA. In patients with FGFR2F, the disease control rate (DCR) was 75.7% (95% CI 66.3–83.6), median progression-free survival (mPFS) was 7.8 months (95% CI 5.5–8.3), and median overall survival (mOS) was 17.2 months (95% CI 12.5–22.4). Patients with FGFR2MA had DCR of 63.6% (95% CI 47.8–77.6), mPFS of 8.3 months (95% CI 3.5–16.7) and mOS of 15.9 months (95% CI 8.4–not evaluable). The most common adverse drug reactions (any grade/grade ≥3) were hyperphosphatemia (35%/4%), asthenia/fatigue (33%/4%), nausea (32%/1%), transaminase elevations (29%/12%), dry mouth (27%/0%) and dry eye (24%/0%). Derazantinib-related nail toxicities (7.5%), retinal events (1.4%), stomatitis (2%) and palmar–plantar erythrodysesthesia (PPE; 1.4%) were rare.
“In patients with FGFR2F, the OS data are comparable to what we have seen before with first-generation FGFR inhibitors. The inclusion of FGFR2MA patients is very interesting,” Braconi comments. “A median OS of 15.9 months in these patients is very promising and could mean that, in the future, we could expand the number of patients who can receive treatment with FGFR inhibitors.”
At the Congress, results were also presented from the first-in-human study of RLY-4008, a highly selective next-generation FGFR2 inhibitor being investigated for the treatment of CCA in FGFR inhibitor-naïve patients with FGFR2 fusions or rearrangements (NCT04526106) (LBA12). In this phase I/II study, the ORR was 63% (24/38; 95% CI 46.0–78.2%) across the doses, with 19 patients having an ongoing response (data cutoff 1 August 2022). In patients who received the recommended phase II dose (RP2D) of 70 mg once daily, the ORR was 88.2% (15/17; 95% CI 63.6–98.5) with an ongoing response in all 15 patients. Median time to response was 1.8 months. The most common treatment-emergent adverse events at the RP2D were low-grade nail toxicities (43%) stomatitis (42%), PPE (35%) and dry mouth (25%).
According to Braconi, “An ORR of 88% is remarkable and higher than the response rates of 20–45% we have seen with other FGFR2 inhibitors. RLY-4008 appears to have good tolerability, with no grade 4 or 5 adverse events reported and no hyperphosphatemia, suggesting this treatment may have a different tolerability profile to other agents in this class. The efficacy and safety findings are very promising, but we need to confirm these results when the data are mature.”
What does the future hold for patients with CCA? “We would like to see if these agents are effective in other groups of patients, such as those who harbour tumours with other alterations of FGFR2 or those who have already progressed with first-line FGFR2 inhibitors,” says Braconi. “So far, treatment with FGFR inhibitors is reserved for patients who have failed chemotherapy, but given the good responses observed, it would be interesting to determine if these agents can be used in the first-line setting and we await the results of ongoing phase III trials,” she concludes.
Abstracts presented:
Borad M, et al. Efficacy of derazantinib in intrahepatic cholangiocarcinoma (ICCA) patients with FGFR2 fusions, mutations or amplifications. ESMO Congress 2022, Abstract 59P
Poster display, Hall 4. An e-Poster is also available on the Congress virtual platform
Hollebecque A, et al. Efficacy of RLY-4008, a highly selective FGFR2 inhibitor in patients (pts) with FGFR2-fusion or rearrangement (f/r), FGFR inhibitor (FGFRi) naive cholangiocarcinoma (CCA): ReFocus trial. ESMO Congress 2022, LBA12
Proffered Paper session 2 – GI, upper digestive, 11.09.2022, h. 14:45 – 16:15, Brest Auditorium