Updated overall survival data from TOPAZ-1 confirm clinically meaningful benefit of adding durvalumab to cisplatin/gemcitabine
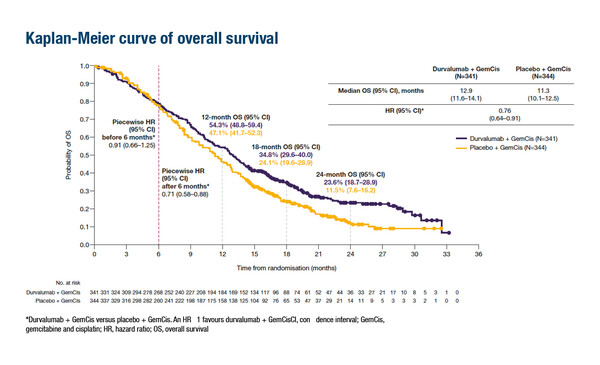
Updated overall survival (OS) and safety data from the randomised phase III TOPAZ-1 study provide support for the addition of durvalumab to cisplatin plus gemcitabine (cisplatin–gemcitabine) in untreated patients with unresectable, locally advanced, recurrent or metastatic biliary tract cancer (BTC). When compared with placebo, the addition of durvalumab to cisplatin-gemcitabine resulted in a longer OS (median [95% confidence interval (CI)] 12.9 [11.6–14.1] months versus 11.3 [10.1–12.5] months, respectively; hazard ratio [HR] 0.76 [95% CI 0.64–0.91]), together with manageable safety (Abstract 56P). When compared with placebo, the addition of durvalumab to cisplatin–gemcitabine resulted in a longer OS (median [95% confidence interval (CI)] 12.9 [11.6–14.1] months versus 11.3 [10.1–12.5] months, respectively; hazard ratio [HR] 0.76 [95% CI 0.64–0.91]), together with manageable safety.
These updated results, presented at the ESMO Congress 2022, confirmed previous findings from the pre-planned interim analysis (NEJM Evid 2022; 1 [8]). Updated OS HRs favoured durvalumab plus cisplatin–gemcitabine in all pre-specified subgroups, including disease status, primary tumour location and PD-L1 positivity. OS rates for durvalumab plus cisplatin–gemcitabine versus cisplatin–gemcitabine, respectively, were 54.3% versus 47.1% at 12 months, 34.8% versus 24.1% at 18 months and 23.6% versus 11.5% at 24 months.
The data were well received by Dr Angela Lamarca from Fundación Jiménez Díaz University Hospital, Madrid, Spain. “The updated data from the TOPAZ-1 trial confirmed the findings of the previous analysis and showed a clinically meaningful benefit of adding durvalumab to cisplatin–gemcitabine compared with cisplatin–gemcitabine alone. Patients with BTC have a very poor prognosis so it is important that we develop better treatment options,” she says commenting on the results. “Unfortunately, most patients are diagnosed late so are only able to receive palliative treatment. The standard of care for many years has been a combination of chemotherapy, mainly cisplatin–gemcitabine, based on the ABC-02 clinical trial (N Engl J Med. 2010;362:1273–1281). But now, based on these results, many guidelines consider adding durvalumab to cisplatin–gemcitabine as standard of care.” Durvalumab plus cisplatin–gemcitabine was recently changed to a category 1, preferred regimen, for the primary treatment of patients with unresectable and metastatic BTC (patients who developed disease recurrence >6 months after surgery with curative intent and >6 months after completion of adjuvant therapy) in the July 2022 update of the NCCN Guidelines (NCCN Guidelines for Hepatobiliary Cancers Version 2.2022).
In addition to the TOPAZ-1 trial data, a second study presented at the annual ESMO Congress also supported the use of durvalumab with cisplatin–gemcitabine in patients with previously untreated BTC or gallbladder cancer. The phase II IMMUCHEC study evaluated the efficacy of two dosing regimens of tremelimumab in combination with durvalumab plus cisplatin–gemcitabine (Abstract 52MO), suggesting no definitive evidence that adding tremelimumab resulted in substantial improvement over durvalumab when one or both was combined with gemcitabine alone or cisplatin–gemcitabine. An inferior outcome was observed in patients who did not receive cisplatin.
“The results of this multi-arm non-comparative phase II study suggest no benefit for using both durvalumab and tremelimumab versus durvalumab alone; the combination of cisplatin–gemcitabine appears to be the best chemotherapy option for combination with durvalumab rather than gemcitabine alone”, noted Lamarca. “In summary, the updated OS data from TOPAZ-1 are more mature and confirm the survival benefit. It is also important to see that this benefit is evident in subgroup analyses and that improved activity is accompanied by a tolerable toxicity profile. The results of the IMMUCHEC study also support this approach. Checkpoint inhibitors are changing the standard of care, and clinical practice guidelines are reflecting this, which is encouraging and very welcome news,” she concluded.
Abstracts presented:
Oh D-Y, et al. Updated overall survival (OS) from the phase 3 TOPAZ-1 study of durvalumab (D) or placebo (PBO) plus gemcitabine and cisplatin (+ GC) in patients (pts) with advanced biliary tract cancer (BTC). ESMO Congress 2022, Abstract 56P
Poster display, Hall 4. An e-Poster is also available on the Congress virtual platform
Vogel A, et al. A randomized phase II trial of durvalumab and tremelimumab with gemcitabine or gemcitabine and cisplatin compared to gemcitabine and cisplatin in treatment-naïve patients with cholangio- and gallbladder carcinoma (IMMUCHEC). ESMO Congress 2022, Abstract 52MO
Mini Oral Session, 10.09.2022, h. 14:45 – 16:15, Toulouse Auditorium