Encouraging clinical activity was reported for fruquintinib and the combination therapy of trastuzumab plus tucatinib in heavily pre-treated patients with manageable safety profiles
Data from the FRESCO-2 and MOUNTAINEER trials, presented at ESMO Congress 2022, provide support for potential new treatment options for patients with previously treated metastatic colorectal cancer (mCRC). FRESCO-2 included a broad population of patients, whereas MOUNTAINEER included only patients positive for HER2 amplification/overexpression.
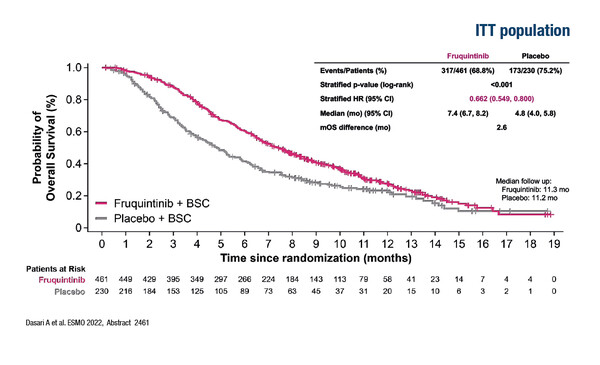
In the phase III FRESCO-2 trial, patients treated with fruquintinib plus best supportive care (BSC; n=461) had a significant and clinically meaningful improvement in overall survival (OS; primary endpoint) compared with those who received placebo plus BSC (n=230) (LBA25). [ADD FIGURE HERE] Median OS was 7.4 months with fruquintinib versus 4.8 months with placebo (hazard ratio [HR] 0.662; 95% confidence interval [CI] 0.549–0.800; p<0.001). Median progression-free survival (PFS) was 3.7 months versus 1.8 months, respectively (HR 0.321; 95% CI 0.267–0.386; p<0.001). Fruquintinib was well tolerated, with safety data consistent with its established profile for monotherapy.
Meeting its statistical endpoint, the trial was well received by Prof. Sebastian Stintzing, from Charité Universitätsmedizin Berlin, Germany, who explains that it evaluated further-line treatment of patients with mCRC, independent of biomarker status. “This study shows the use of tyrosine kinase inhibitors to be beneficial in mCRC, almost doubling OS, which I consider to be the most important endpoint in this setting. This gives us an important treatment option for these patients, with manageable side -effects.” Fruquintinib has already received regulatory approval in China in the third-line onwards mCRC setting, based on data from the FRESCO trial (JAMA. 2018;319:2486–2496; Drugs. 2018;78:1757–1761), and it is anticipated that US FDA approval will be granted soon. “The big question now,” says Stintzing, “is whether we will be able to combine fruquintinib with other approved agents, such as standard chemotherapy or TAS-102 (trifluridine–tipiracil), to try to further improve efficacy while not increasing toxicity.”
New data presented from the phase II MOUNTAINEER study confirmed those shown in the primary analysis at ESMO-World Congress on Gastrointestinal Cancer 2022, showing activity and tolerability of tucatinib plus trastuzumab in patients with HER2-positive mCRC who had received previous treatment (LBA27). The data presented at ESMO Congress 2022 featured a further cohort, Cohort C (n=30), comprising patients who had received tucatinib monotherapy, with the option to cross over to tucatinib plus trastuzumab in the absence of response at week 12 (Cohorts A and B, presented previously, received tucatinib plus trastuzumab). Both tucatinib monotherapy and tucatinib plus trastuzumab after crossover were well tolerated. The objective response rate (ORR) by week 12 in Cohort C was 3.3% (95% CI 0.1–17.2%), with disease control rate (DCR) of 80.0%. Most patients (28/30; 93.0%) crossed over to receive tucatinib plus trastuzumab, with confirmed ORR of 17.9% (95% CI 6.1–36.9%).
Trastuzumab in combination with lapatinib has already demonstrated activity against HER2-positive mCRC in the HERACLES-A trial (Clin Colorectal Cancer. 2020;19:256–262.e2), and the MOUNTAINEER trial now offers a new combination therapy for this patient subgroup, which accounts for approximately 5% of all patients with mCRC (Ann Oncol. 2018;29:1108–1119). “Precision medicine is key for patients with HER2-positive mCRC,”, says Stintzing. “A DCR of 80% after 3 months suggests powerful activity in these patients and this combination appears to be more efficacious than tucatinib monotherapy. Although this is a phase II study that recruited a small number of patients, the findings are optimistic considering the rarity of HER2 positivity and provide support for HER2 testing in this setting.” According to the expert, dual blockade is a promising treatment approach and no new safety concerns were raised in the trial. "However, further data are needed to determine which is the most effective combination for individual patients, which could be trastuzumab–lapatinib, trastuzumab–tucatinib or trastuzumab deruxtecan,“ he concludes.
Abstracts presented:
Dasari NA, et al. FRESCO-2: A global phase III multiregional clinical trial (MRCT) evaluating the efficacy and safety of fruquintinib in patients with refractory metastatic colorectal cancer. ESMO Congress 2022, LBA25
Proffered Paper Session 2 – GI, lower digestive, 12.09.2022, h. 10:15 – 11:45, Fécamp Auditorium
Strickler J, et al. Additional analyses of MOUNTAINEER: A phase II study of tucatinib and trastuzumab for HER2-positive mCRC. ESMO Congress 2022, LBA27
Mini Oral Session – GI, lower digestive, 12.09.2022, h. 14:45 – 15:55, Grenoble Auditorium