Patients with resected stage II–III CRC may benefit from earlier detection of recurrence, enabling more to receive surgery with curative intent, but further work is needed to determine the most reliable and cost-effective methods of follow-up
Data from two clinical trials of the use of biomarkers and imaging to guide treatment decisions and detect recurrence in patients with colorectal cancer (CRC) were reported at ESMO Congress 2022, showing that circulating tumour DNA (ctDNA) may have a place in patient follow-up in the future and that intensive imaging with CT scanning may allow earlier detection of recurrence in patients with resected stage II–III CRC, leading to more curative surgeries.
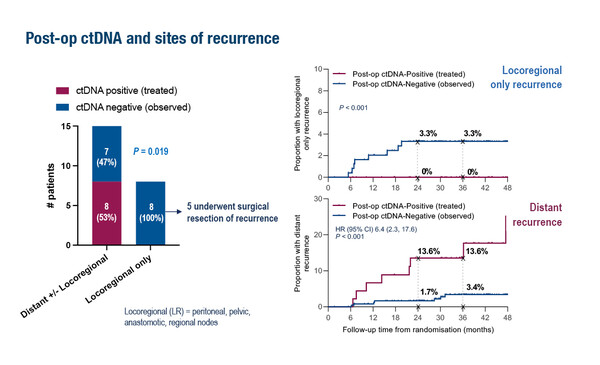
Data presented from the phase II DYNAMIC study followed on from the primary analysis, which showed that giving adjuvant chemotherapy to ctDNA-positive but not ctDNA-negative patients with resected stage II CRC reduced the use of adjuvant chemotherapy without compromising recurrence-free survival (N Engl J Med. 2022;386:2261–2272). The subsequent exploratory analysis presented at ESMO Congress 2022 found that post-operative ctDNA analysis was more sensitive for predicting distant than locoregional recurrences (Abstract 318MO). It also showed that ctDNA clearance could be achieved with adjuvant chemotherapy and this clearance was associated with a positive outcome (2-year recurrence free survival 97%). Neither post-operative nor post-chemotherapy CEA levels added prognostic value for ctDNA-negative patients.
A second presentation reporting on the final results from the phase III PRODIGE 13 trial showed a lack of survival advantage with intensive follow-up (CT scan and CEA) in patients with resected stage II–III CRC when looking at the whole study population. However, intensive follow-up with CT scan provided an increased rate of surgical treatment with curative intent on recurrence (LBA28). These findings confirm those presented at the ESMO Congress 2020 (Ann Oncol. 2020;31(Suppl_4):S409–S461;Abstract 398O).
Data from these two studies were well received by Prof. Julien Taieb, from Université Paris Descartes, France, who explains that, “Adjuvant chemotherapy is used after surgery for CRC to try to prevent recurrence and improve survival. However, it only improves outcomes for ~20% of patients with stage III CRC, which means that 80% of patients experience the associated toxicities but do not benefit. In stage II CRC, only ~5% benefit from this treatment (Ann Oncol. 2020;31:1291–1305). We therefore need to distinguish between patients at greater risk of recurrence who may benefit from adjuvant treatment and those at lower risk who could receive surgery alone.” Prof. Taieb also outlines the economic implications of assessing patients. “Follow-up of patients after surgery and adjuvant treatment is costly; costs vary between countries depending on the frequency of follow-up, although there is a lack of data to support how much follow-up is needed. A more intensive follow-up can identify recurrences earlier, possibly resulting in further surgery or ablative treatment for metastases and cure. As in CRC, some patients with metastatic disease can be cured and more intensive follow-up may allow more patients to be treated with curative intent.”
Although ctDNA testing may avoid the use of chemotherapy in patients unlikely to benefit, Prof. Taieb cautions that more work is needed to ensure the reliability of this approach: “The problem is that we do not know when to use adjuvant chemotherapy in stage II CRC and this varies between sites, despite the publication of the ESMO Guidelines (Ann Oncol. 2020;31:1291–1305). In the DYNAMIC study the relapse rate in ctDNA-negative patients with high-risk factors was not negligible, at around 20%, which is important in this stage II population. Although ctDNA is helpful, we may miss patients who have recurrence but do not have ctDNA, which makes treatment decisions difficult. Countries such as the UK, Netherlands and Australia use a more pragmatic population-based approach whereas the USA and other European countries base decisions on individual patients and are less convinced by population-based data. The data from DYNAMIC showed ctDNA to be a good predictive marker of long-term survival that could be used as a new endpoint for clinical trials as well as changing clinical practice. The classical marker for CRC, CEA, lacks sensitivity and specificity for recurrence and is likely to be replaced by ctDNA in future, but the frequency of monitoring also needs further study.”
“DYNAMIC is a good prospective study, but we now need to confirm these data in larger studies and further research the best technique and approach for ctDNA testing,” says Prof. Taieb. He believes that economic considerations are key: “The cost of ctDNA monitoring is considerably higher than for CEA so further work is needed to examine the cost effectiveness of different methods. It is likely that the cost will reduce over time as ctDNA testing becomes more widely used.”
Prof. Taieb concludes that, “The ability of ctDNA to detect minimal residual disease and to identify appropriate patients for chemotherapy will be the most important tool in the landscape of adjuvant treatment of CRC over the next 10 years. Data from the DYNAMIC trial add to those published from another study showing the prognostic value of serial post-operative ctDNA measurements (Clin Cancer Res. 2022;28:507–517). PRODIGE 13 also provides important information on how best to follow up patients after surgery. An important endpoint is how many patients are eligible for surgery or ablative treatment for recurrence due to a better follow up. The finding that intensive monitoring can increase the rate of curative surgery for recurrence is very important and supports the value of intensive monitoring with CT scans.”
Abstracts presented:
Tie J, et al. Circulating tumour DNA (ctDNA) dynamics, CEA and sites of recurrence for the randomised dynamic study: Adjuvant chemotherapy (ACT) guided by ctDNA analysis in stage II colon cancer (CC) ESMO Congress 2022, Abstract 318MO
Mini Oral Session 2 – GI, lower digestive, 12.09.2022, h. 14:45 – 15:55, Grenoble Auditorium
Lepage C, et al. Prognostic effect of imaging and CEA follow-up in resected colorectal cancer (CRC): Final results and relapse free survival (RFS) - PRODIGE 13 a FFCD phase III trial. ESMO Congress 2022, LBA28
Mini Oral Session 2 – GI, lower digestive, 12.09.2022, h. 14:45 – 15:55, Grenoble Auditorium