Final overall survival results from the KEYNOTE-355 study confirm immune checkpoint inhibitors as the first-line treatment of choice in women with metastatic or relapsing disease
Overall survival results from the KEYNOTE-355 study presented today at the ESMO Congress 2021 support immune checkpoint inhibitors (ICIs) plus chemotherapy as the first-line treatment of choice for patients with PD-L1-positive (CPS ≥10) metastatic triple-negative breast cancer.
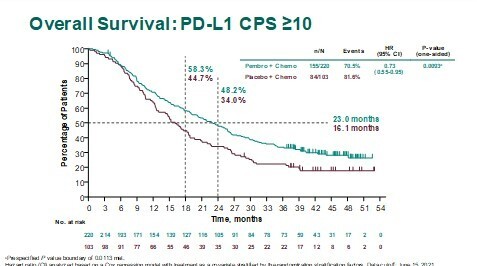
The final results report a longer median overall survival (OS) with first-line pembrolizumab plus chemotherapy versus placebo plus chemotherapy (23.0 months versus 16.1 months, respectively) at a median follow-up of 44.1 months (LBA16). The pembrolizumab combination reduced the risk of death by 27% (hazard ratio 0.73; 95% confidence interval 0.55–0.95; p=0.0093) but no benefit was reported for pembrolizumab plus chemotherapy over chemotherapy by applying a cutoff of CPS ≥1.
This latest report supports the previous findings from the KEYNOTE-355 study that led to its accelerated approval in this setting by the US FDA last year (Lancet. 2020 Dec 5;396(10265):1817-1828). Previously, pembrolizumab plus chemotherapy significantly prolonged progression-free survival (PFS) compared with chemotherapy. “These latest findings are really significant in a disease in which OS has remained poor and unchanged for years,” says Dr Maria Vittoria Dieci from the University of Padua, Italy, commenting on the results. “Due to the mode of action of immunotherapy, the OS gain can often outperform the PFS gain and this is what we saw in KEYNOTE-355 – with advantages for pembrolizumab over chemotherapy of 4 months and 7 months for PFS and OS, respectively. It reflects the results from the IMpassion130 trial, which supported the EMA approval of ICI plus chemotherapy in PD-L1-positive TNBC.” (N Engl J Med 2018; 379:2108-2121).
According to Dieci, research must now focus on better selection of patients and a harmonisation of PD-L1 testing, which is essential to the use of ICIs in this setting. “While we wait for harmonisation, we need to ensure that pathologists and oncologists know which test is needed for which drug. We also need to educate oncologists that the dynamic nature of PD-L1 means that it can change with the evolution of the tumour and site of metastatic disease, so careful selection of biopsy site is essential to determining patient eligibility for immunotherapy.”
Cortés J et al. KEYNOTE-355: Final results from a randomized, double-blind phase 3 study of first-line pembrolizumab + chemotherapy vs placebo + chemotherapy for metastatic TNBC. ESMO Congress 2021, LBA16
Proffered Paper session – Breast cancer, metastatic, 19.9.2021, h. 14:00 – 14:10, Channel 1