Adding an immune checkpoint inhibitor to cetuximab plus radiotherapy does not improve outcomes versus standard of care in locally advanced squamous cell carcinoma of the head and neck
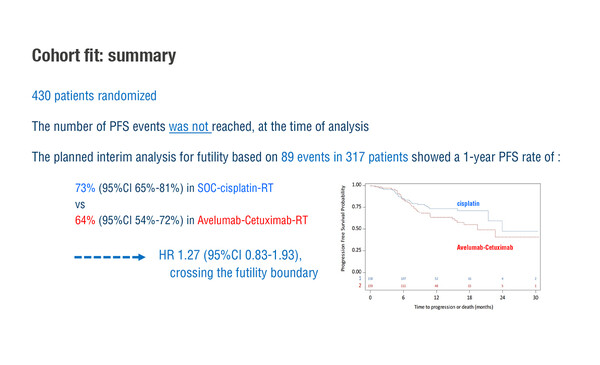
According to results presented yesterday from the phase III GORTEC-REACH study in patients with squamous cell carcinoma of the head and neck (SCCHN), the combination of the anti-PD-L1 antibody avelumab, cetuximab and radiotherapy (RT) did not improve outcomes in those fit for cisplatin compared with standard-of-care intensity-modulated RT [IMRT] plus cisplatin (LBA35). Interim analysis for futility based on 89 events in 317 patients showed 1-year progression-free survival (PFS) rates of 64% for avelumab-based therapy and 73% for standard of care (hazard ratio [HR] 1.27; 95% confidence intervals 0.83–1.93), crossing the futility boundary and favouring standard of care over the ICI-based combination.
Prof. Amanda Psyrri from Attikon University Hospital, Athens, Greece, says these findings indicate that the current standards of care for cisplatin-fit patients will remain unchanged. “It is interesting to observe, however, that the results of the study in cisplatin-fit patients support those from the JAVELIN Head and Neck 100, which was published earlier this year and which compared avelumab plus high-dose cisplatin-based chemoradiotherapy (CRT) with placebo plus high-dose cisplatin CRT in patients with locally advanced SCCHN. In common with the GORTEC-REACH study, the JAVELIN Head and Neck 100 study was stopped at the time of the pre-planned interim analysis after the test statistic crossed the futility boundary based on 224 events.”
The GORTEC-REACH study also demonstrated no benefit for avelumab-based treatment in cisplatin-unfit patients, with a 2-year PFS rate of 44% versus 31% with standard of care (HR 0.84; one-sided p=0.14). “The numerical improvements in PFS and in 2-year locoregional control rate (HR 0.83) with avelumab-based treatment did not reach statistical significance, although there was a statistically significant reduction in the distant metastasis rate (HR 0.31; p=0.007). However, since the primary endpoint of PFS was not met, the standard of care in this population will also remain unchanged,” says Psyrri.
Psyrri speculates on one possible explanation for the disappointing results. “Similarly to the JAVELIN Head and Neck 100 study, the GORTEC-REACH study was conducted in biomarker-unselected patient populations,” she says. “Studies using an enriched population-based strategy may identify the subset of patients with locally advanced HNSCC who derive benefit from incorporation of anti-PD-1 checkpoint inhibitors into standard treatment. An exploratory post-hoc analysis of the JAVELIN Head and Neck 100 study suggests that a potential enrichment factor could be PD-L1; there was a numerical PFS improvement in the avelumab plus CRT arm versus the placebo plus CRT arm in patients with tumours expressing high levels of PD-L1,” observes Psyrri. Other factors, such as radiation delivery, cancer type and the mechanistic interplay between RT and immunotherapy may also affect results, therefore, the sequence of different treatments might be important.
Bourhis, J et al. Avelumab-cetuximab-radiotherapy versus standards of care in patients with locally advanced squamous cell carcinoma of head and neck (LA-SCCHN): Randomized phase III GORTEC-REACH trial. ESMO Virtual Congress 2021, LBA35
Proffered Paper session – Head and neck cancer, excl. thyroid 20.9.2021, h. 13:30 – 13:40, Channel 1