Phase III data highlight the consistent benefit of anti-PD-1 therapy for oesophageal and gastric cancer patients, with greatest benefit in high PD-L1-expressing tumours
At today’s Presidential Symposium, the latest results from the CheckMate-649 study – with an additional 12 months of follow-up – reported a continued improvement of overall survival (OS) with nivolumab plus chemotherapy compared with chemotherapy alone in patients with advanced gastric cancer (GC)/gastroesophageal junction (GEJ) cancer/oesophageal adenocarcinoma and PD-L1 combined positive score (CPS) ≥5 (hazard ratio [HR] 0.70; 95% confidence intervals [CI] 0.61–0.81) (LBA7). In contrast, there was no OS benefit with nivolumab plus ipilimumab compared with chemotherapy in patients with PD-L1 CPS ≥5 (HR 0.89; 95% CI 0.71–1.10; p=0.2302).
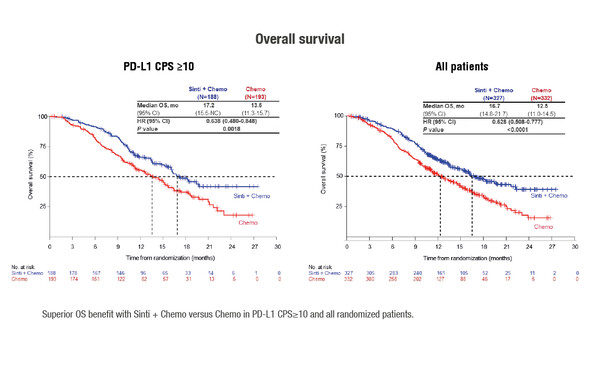
PD-1 inhibition plus chemotherapy also showed remarkable efficacy in three studies conducted in China presented at the ESMO Congress 2021. In the JUPITER-06 trial in advanced or metastatic oesophageal squamous cell carcinoma SCC (ESCC), addition of toripalimab to paclitaxel plus cisplatin (TP) significantly reduced the risk of death (interim OS analysis: HR 0.58; 95% CI 0.43–0.78; p=0.00036) and improved progression-free survival (PFS) (final PFS analysis: HR 0.58; 95% CI 0.46–0.74; p<0.00001) compared with TP alone (Abstract 1373MO). Benefits were observed across all PD-L1 expression groups. First results from the Orient-15 and Orient-16 studies reported findings with sintilimab plus chemotherapy. In Orient-15, sintilimab plus TP or cisplatin/fluoropyrimidine significantly improved OS and PFS in all patients with advanced or metastatic ESCC and those with PD-L1 CPS≥10 (LBA52). In Orient-16, sintilimab plus capecitabine/oxaliplatin significantly improved OS and PFS in patients with gastric/GEJ adenocarcinoma, regardless of PD-L1 expression (LBA53).
“These studies add to the accumulating data showing that chemotherapy plus anti-PD-1 therapy improves outcomes for some groups of patients with oesophageal and gastric cancers,” says Dr Elizabeth Smyth from Addenbrooke's Hospital, Cambridge, UK, putting the findings into context. “It is reassuring to see that there is consistency across European and Asian data sets for these benefits, which also appear to be independent of the chemotherapy regimen used.” She also points to the high response rate reported in the CheckMate-649 study for patients with PD-L1-positive tumours (60% with anti-PD-1 therapy plus chemotherapy) and the generally reasonable tolerability noted across the trials, both of which promise good symptom control and quality of life for patients.
We have a solution for patients whose tumours express PD-L1 at higher levels, but not for those whose tumours are not immune active.
However, Smyth goes on to stress that more work is needed to refine the groups of patients that will derive the most benefit from anti-PD-1 treatment. “What we are seeing, with the trials reported here and with the recently published KEYNOTE 590 trial (Lancet 2021;398:759-771), is that we have a solution for patients whose tumours express PD-L1 at higher levels (CPS ≥5 or ≥10) – but we do not yet have an answer for patients whose tumours are not immune active. We really need more information about what is happening in the subgroup of patients with PD-L1-negative tumours,” she stresses.
Smyth also highlights the need to conduct separate clinical trials for oesophagogastric adenocarcinomas and SCCs, because of their different sensitivities to immunotherapy. “In CheckMate-649, the nivolumab plus ipilimumab combination did not improve survival compared with chemotherapy in patients with adenocarcinomas. This is in contrast to an OS benefit seen with the same combination in the CheckMate-648 study in ESCC (J Clin Oncol 2021;39(Suppl 15); abstr LBA4001).” The appearance of more PD-1 inhibitors in the clinical setting could mean more choice for clinicians. “If there is more than one agent available, the oncologist’s choice will probably be guided by how closely the patient’s profile matches those of patients enrolled in the defining clinical trials, as well as on local regulatory approval and funding decisions. The ESMO Magnitude of Clinical Benefit Scale is useful for informing oncologists and funders about the value benefit of any proposed new treatment,” suggests Smyth.
Looking to the future, Smyth says: “What is really interesting is to move these trials into earlier disease stages – resectable cancers – so that we can cure more patients. The results from trials such as DANTE (J Clin Oncol 2020; 38(15_Suppl):4549; ESMO Congress 2021, abstract 1429P), which is investigating atezolizumab plus fluorouracil/leucovorin/oxaliplatin/docetaxel (FLOT) in resectable oesophagogastric adenocarcinoma, and KEYNOTE 585 (Future Oncol 2019;15:943-952), which is looking at perioperative therapy with or without pembrolizumab for gastric cancer, will provide useful information about immunotherapy in this setting. Combining immunotherapy with different targets, such as HER2 (ESMO Congress 2021, abstract 1379P; J Clin Oncol 2021;39(15_Suppl):4013), also looks promising and future chemotherapy-free combinations would help to reduce toxicity and improve quality of life.”
Janjigian YY. Nivolumab (NIVO) Plus Chemotherapy (Chemo) or Ipilimumab (IPI) vs Chemo as First-Line (1L) Treatment for Advanced Gastric Cancer/Gastroesophageal Junction Cancer/Esophageal Adenocarcinoma (GC/GEJC/EAC): CheckMate 649 Study. ESMO Congress 2021, Abstract LBA7
Presidential symposium 2 19.09.2021, h. 16:12 – 16:27, Channel 1
Shen L. Sintilimab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced or metastatic esophageal squamous cell cancer: First Results of the Phase 3 ORIENT-15 study. ESMO Congress 2021, Abstract LBA52
Mini oral session – Gastrointestinal tumours, non-colorectal 17.09.2021, h. 17:30 – 17:35, Channel 1
Xu J. Sintilimab plus chemotherapy (chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): first results of a randomized, double-blind, phase 3 study. ESMO Congress 2021, Abstract LBA53
Mini oral session – Gastrointestinal tumours, non-colorectal 17.09.2021, h. 17:35 – 17:40, Channel 1
Xu R-H. JUPITER-06: A Randomized, Double-blind, Phase 3 Study of Toripalimab versus Placebo In Combination with First-Line Chemotherapy for Treatment Naive Advanced or Metastatic Esophageal Squamous Cell Carcinoma (ESCC). ESMO Congress 2021, Abstract 1373MO
Mini oral session – Gastrointestinal tumours, non-colorectal 17.09.2021, h. 18:05 – 18:10, Channel 1