Immune chemo-sensitisation results show potential for this approach in the treatment of advanced CRC, but greater understanding of response characteristics is required in future clinical trials
Two phase II trials presented today at the ESMO Congress 2021 report on new immunotherapy-containing approaches to treatment for patients with proficient mismatch repair (pMMR)/microsatellite stable (MSS) metastatic colorectal cancer (mCRC); both met their primary endpoint of progression-free survival (PFS).
A 2-month priming phase with temozolomide, followed by combination immunotherapy with low-dose ipilimumab plus nivolumab was associated with a 36% 8-month PFS rate, and median PFS and overall survival (OS) times of 7.1 months and 18.5 months, respectively, among pre-treated MSS mCRC patients with silencing methylations in the MGMT promoter, according to results of the single-arm MAYA study (Abstract 383O). The results were achieved in the 33 patients who completed the priming phase and were free from progression (24% of the 135 patients who received temozolomide), while the remaining 102 patients had progressive disease or died during priming. The overall response rate (ORR) to the whole strategy was 42%. The vast majority of patients (94%) had received ≥2 previous lines of therapy. Three patients with tumour-paired biopsies showed acquired high tumour mutational burden (TMB). Adverse events (AEs) of any grade (48%) or grade ≥3 (6%) were manageable with protocol guidelines.
A detailed analysis of response characteristics and further exploration will be useful for gauging the true effect of temozolomide immune sensitisation in this setting.
“The increased ORR achieved in comparison to that reported with single-agent temozolomide in similar populations, and the 36% PFS rate at 8 months in the context of the reported increases in TMB look very promising and may point to synergistic effects,” says Dr Guillem Argilés from Memorial Sloan Kettering Cancer Center, New York, NY, USA. He adds: “However, the possibility remains that the efficacy observed is a result of the population being highly selected due to the 2-month priming phase, given that only one-quarter of the patients entering the priming phase were able to complete it. It could be that the patients with less aggressive disease were those who completed the priming without progressing and this impacted on PFS figures. A detailed analysis of response characteristics and further exploration, including an appropriate control arm, will be really useful for gauging the true effect of temozolomide immune sensitisation in this setting.” Biomarkers are going to be crucial for the effective use of regimens, such as the one used in the MAYA trial, according to Argilés: “Analysis of biomarkers and their correlation with response will be key to identifying which patients are likely to respond to treatment. Biomarkers are needed at the very outset, so that patients unlikely to respond to priming are spared exposure to the primer and its associated toxicities and instead, they are offered alternative treatment to optimise their likelihood of a good outcome.”
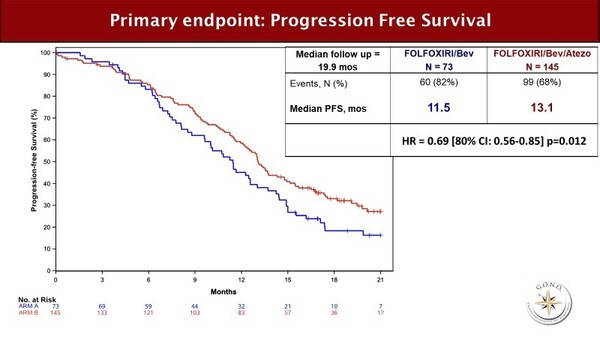
In the randomised AtezoTRIBE trial, the use of atezolizumab to consolidate the activity of FOLFOXIRI plus bevacizumab led to a 1.6-month increase in PFS compared with FOLFOXIRI plus bevacizumab in front-line mCRC (13.1 months versus 11.5 months; hazard ratio [HR] 0.69; 80% confidence interval [CI] 0.56–0.85; p=0.012) (LBA20) (Figure). When the analysis was restricted to the pMMR group (91% of patients) the PFS figures were 12.9 months versus 11.4 months; HR 0.78; 80% CI 0.62–0.97; p=0.071). Rates of grade 3–4 AEs were similar between arms; immunotherapy-related AE rates were 3% with atezolizumab and 1% without atezolizumab.
The combination set a landmark PFS of 13.1 months for front-line mCRC, without impairing tolerability. “FOLFOXIRI plus bevacizumab is already a regimen for selected patients, so it is encouraging to see that combining it with atezolizumab did not appear to increase the rates of all-grade or severe AEs,” comments Argilés. “However, it will be important to see what both the efficacy and the toxicity profiles of this combination look like in a real-world setting, with a less well-selected patient population.” Also, the cost and the complexity of the combination, particularly when administered outside a clinical trial setting, could pose additional barriers to the widespread use of FOLFOXIRI plus bevacizumab plus atezolizumab.
“Although the real clinical impact of these two different strategies is still pending demonstration in more mature clinical trials, I am pleased because they pave the way for immune-sensitisation in advanced MSS mCRC,” concludes Argilés.
Pietrantonio F et al. MAYA trial: temozolomide (TMZ) priming followed by combination with low-dose ipilimumab and nivolumab in patients with microsatellite stable (MSS), MGMT silenced metastatic colorectal cancer (mCRC). ESMO Congress 2021, Abstract 383O
Proffered Paper session – Gastrointestinal tumours, colorectal 1, 18.09.2021, h. 13:30 – 14:50, Channel 2
Cremolini C et al. FOLFOXIRI plus bevacizumab (bev) plus atezolizumab (atezo) versus FOLFOXIRI plus bev as first-line treatment of unresectable metastatic colorectal cancer (mCRC) patients: results of the phase II randomized AtezoTRIBE study by GONO. ESMO Congress 2021, Abstract LBA20
Proffered Paper session – Gastrointestinal tumours, colorectal 1, 18.09.2021, h. 13:30 – 14:50, Channel 2