Good response rate and disease control with adagrasib and sotorasib in combination therapy shown in early-stage trials
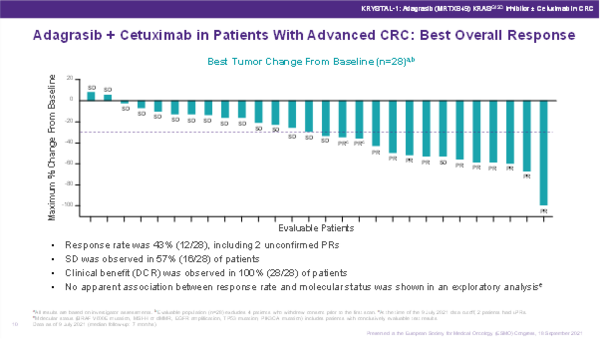
At the ESMO Congress 2021, two presentations of preliminary data demonstrated the promising activity of specifically designed KRAS G12C inhibitors. As part of the KRYSTAL-1 phase I/II trial, adagrasib was investigated as monotherapy (n=46) and in combination with cetuximab (n=32) in heavily pre-treated patients with KRAS G12C-mutated colorectal cancer (CRC) (LBA6). Adagrasib monotherapy among 45 evaluable patients resulted in an overall response rate (ORR) of 22% and disease control rate (DCR) of 87%, while the combination of adagrasib and cetuximab produced even more promising results among 28 evaluable patients: an ORR of 43% and DCR of 100%.
In a second study, the phase Ib CodeBreaK101 trial, another new KRAS G12C inhibitor, sotorasib, was combined with panitumumab in a similar population of 31 chemorefractory patients (Abstract 424P). Confirmed ORR was 15%, with a confirmed plus unconfirmed ORR of 27% and DCR of 81%.
Dr Rodrigo Dienstmann from Grupo Oncoclinicas, San Paulo, Brasil, is very encouraged by these findings. “Although patients with KRAS G12C mutations represent a relatively small population of patients with metastatic CRC (mCRC) – around 3–4% of the total – these patients have more aggressive disease with a poor prognosis and there are no targeted therapies. The response rates and disease control seen in these trials may well translate into improved survival,” he says.
Dienstmann believes that combinatorial approaches are critical with these new agents. In fact, results observed with the KRAS G12C inhibitors are in line with preclinical evidence suggesting that there is still dependence on the EGFR pathway upstream when you block the MAPK pathway downstream in KRAS-mutant CRC. “It is likely that use of a KRAS G12C inhibitor as a single agent will only be justified in lung cancer,” he continues. “Real-world data are currently being collected on sotorasib, since it has been already approved as single agent for KRAS G12C-mutated lung cancer in the USA. Additional data may help with further clinical development, particularly related to the safety profile, which currently appears acceptable based on initial data.”
Further studies are now ongoing with adagrasib and sotorasib as second-line treatment, which will help to better understand where these inhibitors should be positioned in the therapeutic journey of patients with KRAS G12C mutant CRC.
Less conclusive results – this time in stable chemo-responsive CRC – were shown from the phase II FOCUS4-C trial with adavosertib, an inhibitor of the cell-cycle checkpoint regulator WEE1 kinase (Abstract 382O). In total, 69 patients with newly diagnosed mCRC, and TP53 and RAS mutations, who were stable or responding after 16 weeks of chemotherapy were randomised 2:1 to receive adavosertib or active monitoring.
In the study, there was significant improvement of the primary outcome, progression-free-survival (PFS), with adavosertib versus active monitoring (median 3.61 months versus 1.87 months, respectively; hazard ratio [HR] 0.35; 95% confidence interval [CI] 0.18–0.68; p=0.0022). However, median overall survival was not improved with adavosertib compared with active monitoring (13.1 months versus 11.3 months, respectively; HR 0.86; 95% CI 0.46–1.62; p=0.65). In a pre-specified subgroup analysis, adavosertib activity appeared to be restricted to left-sided tumours (left sided: HR 0.24; 95% CI 0.11–0.51 versus right-sided: HR 1.02; 95% CI 0.41–2.56; interaction p=0.043).
Commenting on these findings, Dienstmann says, “There is a signal of antitumour activity with prolonged PFS, but the absolute improvement is less than 2 months compared with surveillance. However, the limited activity in right-sided tumours is not surprising.” Future research is needed to clarify whether adavosertib combination approaches may help extend the overall findings and may be able to circumvent the intrinsic resistance of right-sided CRC to targeted therapies.
“WEE1 kinase inhibitors are being investigated as combination therapies in TP53-mutant and SET domain-containing 2 deficient (SETD2) malignancies, with promising results in ovarian cancer, for example,” concludes Dienstmann. “It will be interesting to see if the results of FOCUS4-C will translate into a phase III trial with adavosertib, whether this trial will involve combination therapy and if the maintenance setting will be prioritised.”
Weiss J et al. KRYSTAL-1: Adagrasib (MRTX849) as monotherapy or combined with cetuximab (Cetux) in patients (Pts) with colorectal cancer (CRC) harboring a KRASG12C mutation. ESMO Congress 2021, Abstract LBA6
Presidential symposium 2, 19.9.2021, h. 1547 – 16:02, Channel 1
Fakih M et al. CodeBreaK 101 subprotocol H: Phase Ib study evaluating combination of sotorasib (Soto), a KRASG12C inhibitor, and panitumumab (PMab), an EGFR inhibitor, in advanced KRAS p.G12C-mutated colorectal cancer (CRC). ESMO Congress 2021, Abstract 424P
Seligmann J et al. Inhibition of WEE1 is effective in TP53 and RAS mutant metastatic colorectal cancer (mCRC): A randomised phase II trial (FOCUS4-C) comparing adavosertib (AZD1775) with active monitoring. ESMO Congress 2021, Abstract 382O
Proffered Paper session – Gastrointestinal tumours, colorectal 1, 18.9.2021, h. 13:30 – 13:40, Channel 2