Trials provide insights into perioperative and adjuvant chemotherapy for resectable pancreatic cancer
Results presented in a Proffered Paper session today suggest that while there appear to be positives associated with the use of perioperative chemotherapy for resectable pancreatic ductal adenocarcinoma (PDAC), there are still some important unknowns (LBA56).
In the NEONAX phase II trial, a higher proportion of patients in the perioperative group were able to complete treatment (70% of patients started neoadjuvant chemotherapy and were R0/R1 resected), compared to the adjuvant arm (42% of patients underwent R0/R1 resection and started adjuvant chemotherapy). The trial randomised 127 patients with resectable PDAC to receive perioperative chemotherapy (2 cycles of nab-paclitaxel/gemcitabine followed by tumour surgery and 4 cycles of nab-paclitaxel/gemcitabine) or adjuvant chemotherapy (tumour surgery followed by 6 cycles of nab-paclitaxel/gemcitabine).
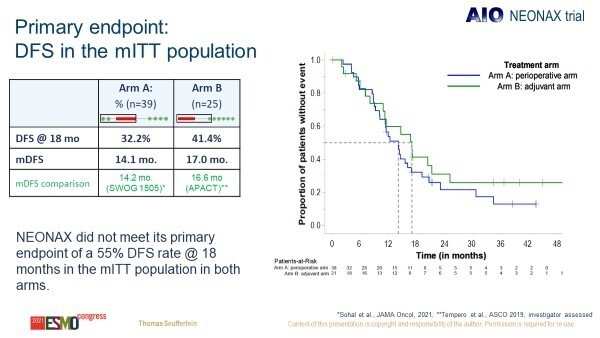
Resection rates were similar between arms (69.5% perioperative arm versus 78.0% adjuvant arm) but R0 resection rates were higher in the perioperative group (87.8%) compared with the adjuvant group (67.4%). Despite these potential benefits in favour of perioperative approaches, the primary endpoint, improvement in disease-free survival (DFS) at 18 months in at least one arm to ≥55%, was not met (reported DFS rate was 32.2% in the perioperative group and 41.4% in the adjuvant group) (Figure 1).
We still do not have conclusive evidence that perioperative chemotherapy actually improves outcomes.
“Findings from NEONAX indicate that perioperative chemotherapy is deliverable and that higher R0 resection rates may be achieved by utilising such strategies. However, this did not translate into a survival benefit. This was not wholly unexpected, and it has also been the case in some other prior studies in the field,” says Dr Angela Lamarca from The Christie NHS Foundation Trust and the University of Manchester, UK, commenting on the data. “The phase III PREOPANC trial (J Clin Oncol. 2020 Jun 1; 38(16): 1763–1773) also failed to demonstrate the survival benefits of preoperative chemoradiotherapy for resectable PDAC, so we still do not have conclusive evidence that perioperative chemotherapy actually improves outcomes.”
In addition, she thinks that interpretation of the NEONAX trial data is somehow limited by the choice of adjuvant chemotherapy schedule. “We know from the APACT trial (Annals of Oncology 30 (Supplement 4): iv126–iv136, 2019) that the control arm tested in NEONAX, nab-paclitaxel plus gemcitabine, does not improve survival following surgery – it is not the standard of care. So, while I think that perioperative treatment does potentially have a role to play in resectable disease, further studies will be required to conclusively demonstrate survival benefit and determine the most appropriate perioperative treatment.”
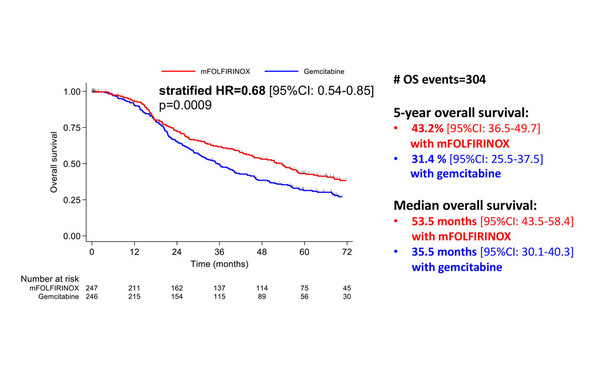
The same session revealed updated results from the PRODIGE 24/CCTG PA6 phase III trial comparing a modified FOLFIRINOX (mFOLFIRINOX) regimen with gemcitabine as adjuvant therapy in patients with resected PDAC (LBA57). The 5-year DFS rate was 26.1% with mFOLFIRINOX versus 19.0% with gemcitabine (stratified hazard ratio [HR] 0.66; 95% confidence interval [CI] 0.54–0.82; p=0.0001), while median overall survival (OS) was 53.5 months in the mFOLFIRINOX arm versus 35.5 months in the gemcitabine arm (stratified HR 0.68; 95% CI 0.54–0.85; p=0.0009) (Figure 2). “We know that FOLFIRINOX is the new standard of care in the adjuvant setting,” explains Lamarca, “but now we have supportive 5-year data showing improvement in both DFS and OS, with clinically meaningful differences in favour of mFOLFIRINOX.”
Another novel finding is that treatment arm, centre volume of inclusions, tumour grade, tumour staging and patient age are significant prognostic factors for OS in multivariate analysis. Lamarca says, “It is notable that centre volume was a significant factor for survival. Surgery for pancreatic cancer is not an operation that can be done at any centre – a lot of experience in the type of resection is needed and this is a very important point to make.”
Looking to the future, Lamarca thinks that further studies are needed to identify biomarkers to select patients in whom adjuvant 5-fluorouracil-based (over gemcitabine-based) therapy is likely to be most effective, especially for patients who may not be well enough for triplet chemotherapy.
Seufferlein T et al. Perioperative or only adjuvant nab-paclitaxel plus gemcitabine for resectable pancreatic cancer - results of the NEONAX trial, a randomized phase II AIO study. ESMO Congress 2021, Abstract LBA56
Proffered Paper session - Gastrointestinal tumours, non-colorectal, 19.9.2021, h. 13:30 – 13:40, Channel 4
Conroy T el al. Unicancer PRODIGE 24/CCTG PA6 trial: Updated results of a multicenter international randomized phase 3 trial of adjuvant mFOLFIRINOX (mFFX) versus gemcitabine (gem) in patients (pts) with resected pancreatic ductal adenocarcinomas (PDAC). ESMO Congress 2021, Abstract LBA57
Proffered Paper session - Gastrointestinal tumours, non-colorectal, 19.9.2021, h. 13:40 – 13:50, Channel 4