Preliminary data indicate tolerability and disease control with bexmarilimab in hard-to-treat solid tumours
Bexmarilimab is a novel humanised antibody that targets Clever-1, a macrophage scavenger receptor known to induce intratumoural immunosuppression. As presented today at the ESMO Congress 2021, the first-in-human three-part phase I/II MATINS trial is ongoing to assess the safety and preliminary efficacy of bexmarilimab in patients with advanced solid tumours (LBA38).
A total of 30 patients have completed part 1 of the trial (dose: 0.1–10 mg/kg), while 110 patients have been assessed in part 2 (dose: 0.3–3.0 mg/kg every 3 weeks). Enrolled patients were all heavily pre-treated, refractory to all other beneficial treatments and had 11 different types of metastatic cancer.
The most common treatment-emergent adverse events with bexmarilimab were fatigue (41%), anaemia (25%) and abdominal pain (24%). In part 2, there were 13 treatment-related grade 3-4 adverse events and no treatment-related grade 5 adverse events.
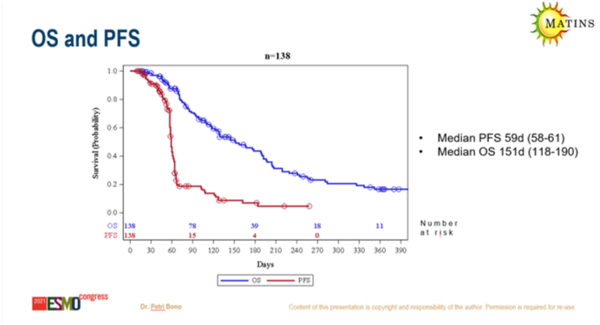
In a survival analysis of patients in part 1 and part 2 (n=138), median progression-free survival was 59 days (95% confidence interval [CI] 58–61) and median overall survival was 151 days (95% CI 118–190).
In part 2, the clinical benefit rate (CBR) was 17%, with 19 out of 110 patients having stable disease. A promising CBR of 30–40% was noted in patients with cutaneous melanoma, cholangiocarcinoma, gastric cancer, hepatocellular carcinoma and oestrogen receptor-positive breast cancer at cycle 4.
The MATINS trial continues, and further investigations will focus on optimal dosing, identifying biomarkers of efficacy and the potential for combining bexmarilimab with other therapies during earlier lines of therapy.
Bono P et al. Bexmarilimab, a novel macrophage re-programmer shows promising anti-tumour activity in phase I/II trial in several last line solid tumour types. ESMO Congress 2021, Abstract LBA38
Proffered Paper session – Investigational immunotherapy, 17.9.2021, h. 13:30 – 13:40, Channel 4