Mixed results from the the HERACLES-B, TRIUMPH and MOUNTAINEER trials
There were mixed results yesterday for dual-targeted therapy in HER2-amplified KRAS wild-type metastatic colorectal cancer (mCRC). The HERACLES-B trial (Abstract LBA35) did not reach its primary endpoint but encouraging objective response rates (ORRs) were reported from the TRIUMPH sub-study, GOZILA (Abstract 526PD) and the MOUNTAINEER trial (Abstract 527PD).
In the open-label phase II HERACLES-B trial of pertuzumab plus trastuzumab emtansine (T-DM1) (n=30), the ORR was 10%. However, many patients (70%) did experience stable disease and higher HER2 immunohistochemistry scores were associated with objective response/stable disease ≥4 months (p=0.03). Dr Elizabeth Smyth from Cambridge University Hospitals NHS Foundation Trust, UK, provided her insight into the results: "It is unclear why response rates are lower than expected in HERACLES-B,” she says comparing these findings with the ORR of 30% in the HERACLES-A with trastuzumab and lapatinib reported some years ago.
"T-DM1 used in HERACLES-B combines a cytotoxic payload with trastuzumab and it would be reasonable to expect higher response rates than with the non-cytotoxic combination of trastuzumab plus lapatinib used in HERACLES-A. However, it is possible that mCRC is less sensitive to emtansine chemotherapy—which is a microtubulin inhibitor—than breast cancer, the disease in which T-DM1 has previously been shown to be effective. Alternatively, it could be that blocking EGFR with lapatinib was also helpful for these tumours.”In contrast to HERACLES-B, GOZILA and MOUNTAINEER achieved their primary endpoints, with ORRs ≥30%. "Considering that the patients enrolled in GOZILA and MOUNTAINEER were previously chemorefractory, the results are impressive,” says Smyth. "Response rates to second-line chemotherapy in mCRC are around 20% and these results for anti-HER2 therapy appear to exceed that, although we need to be cautious not to over-interpret the results of small datasets.
”In the GOZILA sub-study, HER2 amplification was confirmed by analysis of tumour tissue and/or circulating tumour DNA (ctDNA): 14 patients had HER2 amplification in both tissue and ctDNA; HER2 amplification was confirmed by tissue alone in 3 patients and in ctDNA alone for 1 patient. Investigators found that trastuzumab plus pertuzumab was associated with an ORR of 35% in tissue-positive patients and 33% in ctDNA-positive patients. The antitumor activity seen in GOZILA is in line with the results from the MyPathway study investigating the same combination in HER2-amplified mCRC by tissue profiling. [2]"It is reassuring that there was substantial overlap when HER2 amplification was assessed in ctDNA and tissue, indicating that either method could be used for patient selection,” remarks Smyth. "Additionally, the efficacy of trastuzumab plus pertuzumab was similar for both methods, which suggests that there is no difference in the sensitivity of ctDNA- or tissue-detected HER2-positive mCRC to anti-HER2 therapy. Liquid biopsy, or measurement of ctDNA in plasma could, in future, decrease the need for tissue confirmation of HER2 status.”
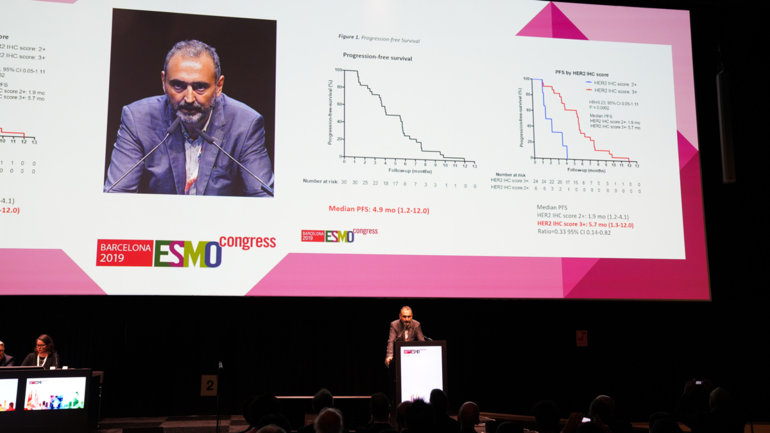
In the MOUNTAINEER trial, the ORR was 55% in the 22 evaluable patients who received tucatinib plus trastuzumab. Furthermore, median progression-free survival was 6.2 months and median overall survival (OS) was 17.3 months. Smyth is excited by these findings. "This is a very encouraging response rate for a novel HER2 tyrosine kinase inhibitor and antibody combination,” she says. "Unlike trastuzumab and pertuzumab, tucatinib crosses the blood–brain barrier and has the potential to treat brain metastases, which can occur even in patients with good systemic disease control who are treated with anti-HER2 therapies.”
"The median OS of 17 months in a chemorefractory population shows the power of treating this sensitive population with the right specifically targeted therapies,” highlights Smyth."In future, moving anti-HER2 therapy to earlier lines of mCRC treatment may lead to even higher response rates. We also need to consider the best time to measure HER2 amplification in mCRC. Is the amplification an inherent tumour characteristic or is it acquired, for example, after anti-EGFR therapy? Is response to anti-HER2 therapy different in these two scenarios? Also, as HER2 overexpression seems to predict worse outcomes in RAS wildtype patients treated with anti-EGFR therapy, should we be using HER2 overexpression as a negative selection biomarker for anti-EGFR therapy in mCRC? This is the topic of much debate.”And to conclude, Smyth says, "It is challenging to work on trials where the genetic abnormality is rare, but if we consider NTRK, ALK or ROS1, and now HER2 amplification, we can see what is possible. Results from these phase II trials suggest that in the next few years, we will be able to treat patients with HER2-positive mCRC with the right agents, and possibly even with chemotherapy-free combinations—this is great news for patients.”
ESMO Congress 2019 abstracts:
- LBA35 - Phase II study of pertuzumab and trastuzumab-emtansine (T-DM1) in patients with HER2-positive metastatic colorectal cancer: The HERACLES-B (HER2 Amplification for Colo-rectaL cancer Enhanced Stratification, cohort B) trial
- 526PD - TRIUMPH: Primary efficacy of a phase II trial of trastuzumab (T) and pertuzumab (P) in patients (pts) with metastatic colorectal cancer (mCRC) with HER2 (ERBB2) amplification (amp) in tumour tissue or circulating tumour DNA (ctDNA): A GOZILA sub-study
- 527PD - Trastuzumab and tucatinib for the treatment of HER2 amplified metastatic colorectal cancer (mCRC): Initial results from the MOUNTAINEER trial