The management of biliary tract cancers, especially cholangiocarcinoma, is rapidly changing with emerging new precision medicine strategies
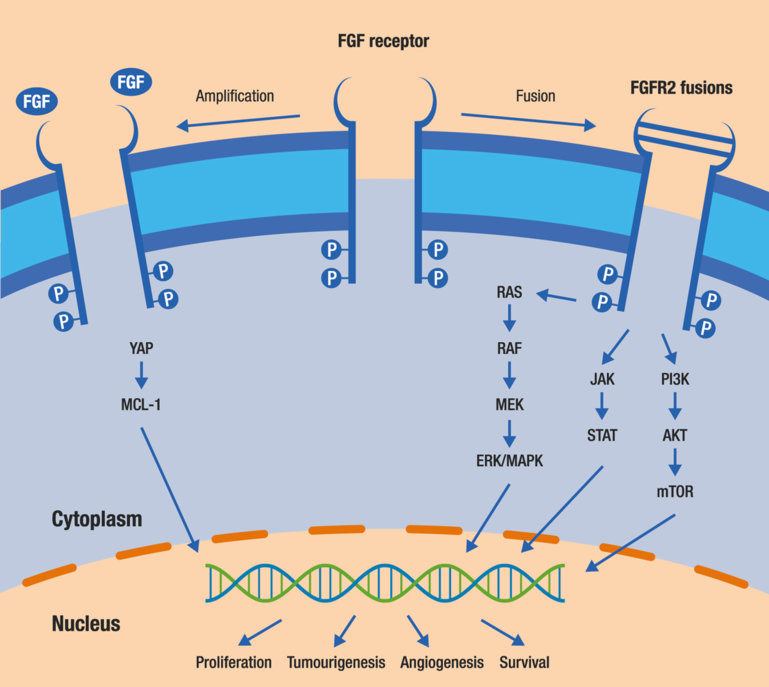
"Most research is focused on patients harbouring fibroblast growth factor receptor-2 (FGFR2) fusions and isocitrate dehydrogenase-1 (IDH1) mutations, which could be present in up to approximately 40% of patients with intrahepatic cholangiocarcinoma, therefore screening all patients with cholangiocarcinoma could be a key factor in helping clinicians to choose the most appropriate treatment pathway and improve patient outcomes,” says Dr Angela Lamarca from The Christie NHS Foundation Trust, Manchester, UK. Late-Breaking Abstract results from the FIGHT-202 study presented at ESMO 2019 show that in patients with pretreated, advanced cholangiocarcinoma, pemigatinib therapy is associated with clinically meaningful efficacy (Abstract LBA40).
Pemigatinib is a FGFR inhibitor and in this study, response to treatment was restricted to patients with FGFR2 gene rearrangements or fusions. The objective response rate (ORR) was 35.5%, including three complete responses and a median response duration of 7.5 months. The disease control rate (DCR) was 82%. Median progression-free survival (PFS) and overall survival were 6.9 months and 21.1 months, respectively. The most common adverse event was grade 1–2 hyperphosphatemia (incidence 60%), which was managed primarily with diet modifications and the administration of phosphate binders. Results from an abstract submitted separately suggest that the specific rearrangement partner does not impact on response to therapy (Abstract 720P).Clinical efficacy was demonstrated for another FGFR2 inhibitor, derazantinib, in a non-comparative phase IIa study (Abstract 721P).
Similar to the findings from FIGHT-202, the efficacy of derazantinib was more pronounced in the presence of FGFR2 aberrations: ORR 21%.Commenting on the results, Lamarca says, "It is encouraging to see consistently high response rates with the various FGFR inhibitors that have been tested; a meaningful, actionable target for patients with cholangiocarcinoma seems to have been identified.”
"Research is moving fast in the field of cholangiocarcinoma, with efforts not only attempting to identify active treatments, but also trying to understand the clinical implications of these genetic alterations,” explains Lamarca. Patients with FGFR2 fusions appear to have a significant survival advantage compared with those without FGFR fusions (Abstract 726P) and new research findings point to younger age (<65 years), alcohol abuse and hepatitis B or C positivity increasing the likelihood of FGFR2 fusions being present (Abstract 723P)." Tumour profiling should become a new standard for patients diagnosed with advanced cholangiocarcinoma,” says Dr Angela Lamarca from The Christie NHS Foundation Trust, Manchester, UK. There is continuing progress in the cholangiocarcinoma setting, including research into new first-line agents. For instance, in the ongoing PROOF study, the pan-FGFR inhibitor infigratinib is being compared with standard-of-care therapy with gemcitabine plus cisplatin in patients with advanced/metastatic or inoperable cholangiocarcinoma (Abstract 832TiP).
"Next, we need to understand how active are these agents compared with our current standard-of-care chemotherapy and where to place them in the patient pathway,” pointed out Lamarca. "For the first time, results at ESMO Congress 2019 demonstrate that a greater understanding of the genomic landscape of cholangiocarcinoma is leading to investigational targeted therapies showing great promise in clinical trials, but only when given to the right patients,” says Lamarca. She concludes: "Precision medicine has been a reality in lung cancer and other areas of oncology for many years and with these new data, it is only now that we dare to think of it as a possibility in cholangiocarcinoma.”
ESMO Congress 2019 abstracts:
- LBA40 - FIGHT-202: A phase II study of pemigatinib in patients (pts) with previously treated locally advanced or metastatic cholangiocarcinoma (CCA)
- 721P - Efficacy of derazantinib (DZB) in patients (pts) with intrahepatic cholangiocarcinoma (iCCA) expressing FGFR2-fusion or FGFR2 mutations/amplifications
- 723P - Frequency and clinicopathological characteristics of biliary tract carcinomas harboring the FGFR2-fusion gene: A prospective observational study (PRELUDE study)
- 832TiP - Infigratinib versus gemcitabine plus cisplatin multicenter, open-label, randomized, phase III study in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: The PROOF trial