The COSMIC-312 trial met its primary endpoint of progression-free survival, but interim overall survival results need further exploration
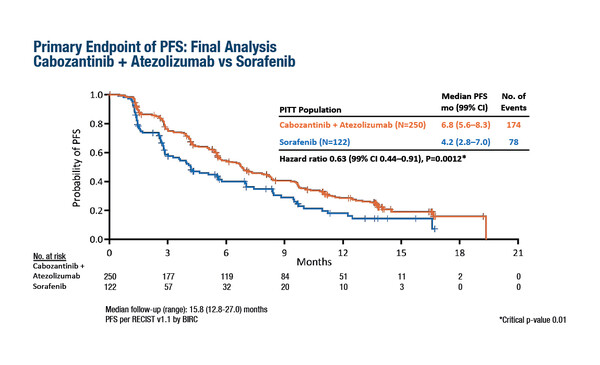
A presentation in today’s ESMO Virtual Plenary at the ESMO Asia Virtual Oncology Week 2021 reports that a combination of the multikinase inhibitor cabozantinib with the immune checkpoint inhibitor atezolizumab significantly improved progression-free survival (PFS) compared with sorafenib alone (hazard ratio [HR] 0.63, 99% confidence interval [CI] 0.44–0.91; p=0.0012) in the first-line treatment of advanced hepatocellular carcinoma (HCC) (Abstract VP10-2021). In the global phase III, open-label COSMIC-312 trial, the median PFS was 6.8 months with cabozantinib + atezolizumab and 4.2 months with sorafenib (Figure). However, a prespecified interim overall survival (OS) analysis showed no significant benefit for the immunotherapy combination over sorafenib (HR 0.90, 96% CI 0.69–1.18; p=0.438).
The trial randomised 837 patients with advanced HCC 2:1:1 to receive cabozantinib + atezolizumab, sorafenib alone or cabozantinib alone. Randomisation was stratified by aetiology, geographic region and presence of extrahepatic disease and/or microvascular invasion. Altogether, 30% of patients had hepatitis B virus (HBV), 31% had hepatitis C virus (HCV) without HBV and 39% had non-viral aetiology. Over one-quarter of patients (29%) were enrolled in Asia. Grade 3–4 treatment-related adverse events occurred in 54% of patients receiving cabozantinib + atezolizumab and in 32% receiving sorafenib.
“The PFS benefits in COSMIC-312 are very similar to those achieved in IMbrave150, presented at the ESMO Asia Congress 2019, which signalled the benefit of immunotherapy in advanced HCC and led to atezolizumab + bevacizumab becoming first-line standard of care in this setting,” remarks Dr Angela Lamarca The Christie NHS Foundation Trust, Manchester, UK (N Engl J Med. 2020;382:1894–1905). In the IMbrave150 trial, median PFS was 6.8 months and 4.3 months with atezolizumab + bevacizumab and sorafenib, respectively (HR 0.59). However, the interim OS analysis results in COSMIC-312 are problematic. “We need to better understand why the benefit in PFS is not translated into a proportional OS benefit, which was the case in IMbrave150,” she adds. “One possible factor could be the subsequent therapies received by patients in the control arm of COSMIC-312. For example, did patients progressing on sorafenib go on to receive immunotherapy? Another factor could be differences in baseline aetiologies of HCC, which we know from IMbrave150 may impact on the benefit of immunotherapy-based treatment.”
This is something that is particularly pertinent for Asia-Pacific, where HCC is more common than in other parts of the world and where it is caused predominantly by HBV, compared with HCV and non-viral causes (Gut Liver. 2016;10:332–339). More information on biomarkers predictive of response and resistance will also be crucial to define the patient selection criteria for immunotherapy-based treatment for HCC. “The findings from COSMIC-312 appear to further support the use of immunotherapy in the first-line treatment of advanced HCC and could provide physicians with an additional treatment option,” says Lamarca, “but this all may depend on final OS and secondary end-point data.”
Kelley RK et al. Cabozantinib (C) plus atezolizumab (A) versus sorafenib (S) as first-line systemic treatment for advanced hepatocellular carcinoma (aHCC): results from the randomized phase 3 COSMIC-312 trial. ESMO Asia Virtual Oncology Week 2021, VP10-2021
ESMO Asia Virtual Oncology Week 2021, Virtual Plenary, 20.11.2021, h. 19:10 – 19:25 (Singapore time), Channel 1